INTRODUCTION
The annual report in 2020 indicated a high prevalence of breast cancer, cervical cancer, and colorectal cancer worldwide (American Cancer Society, 2020). The development of monocarbonyl analogs of curcumin to be tetrahydro-indazole structures showed good antioxidant and antitumor against Michigan Cancer Foundation (MCF-7), WI38, Hep G2, and vero cells (Bayomi et al., 2015; Bayomi et al., 2013). Indazole compounds are scarcely found in nature, generally prepared by organic synthesis. The formation of the indazole ring significantly improved the biological activity of the molecules (Gaikwad et al., 2015; Plescia et al., 2010; Shrivastava et al., 2016; Thangadurai et al., 2012; Thirupalu et al., 2014; Zhang et al., 2018). Recently, in silico studies using pharmacophore modeling and docking methods indicated that several asymmetric hexahydro-2H-indazoles were potentially active as ERα inhibitors (Hariyanti et al., 2021). Therefore, we prepared six novel curcumin indazole analogs and evaluated them for their cytotoxicity against breast (MCF-7), cervical (HeLa), and colorectal (WiDr) cancer cells. To evaluate their selectivity, the compounds’ cytotoxicity was also tested against normal vero cells.
MATERIALS AND METHODS
General procedures
All chemicals (E. Merck, Germany, or Sigma-Aldrich, USA) were obtained commercially. Purity tests and reactions monitoring were carried out using the thin layer chromatography procedure. Melting points (m.p.) were assessed by the melting point instrument (Bibby Sterilin, UK) and were not corrected. Infrared (IR) spectra were scanned in a Kalium Bromide mixture on the Shimadzu FTIR-8400S Spectrometer (Japan). Nuclear magnetic resonance (NMR) spectra were run in a CDCl3 solution on a JEOL JNM 500 Spectrometer (Peabody, USA). Mass spectra were found using electrospray ionization (+) mode UNIFI-Waters Liquid Chromatography-Mass Spectrometry (MS)/MS (USA). 3–(4–Methoxyphenyl/3,4-dimethoxyphenyl)–3,3a,4,5,6,7–hexahydro–2H–indazole (1a-b), cyclovalone (4a), and 2,6-bis-[(E)-4-hydroxybenzylidene]cyclohexanone (4b) used as starting materials were prepared according to the reported method (Hayun et al., 2017; Minu et al., 2009; Rahmawati et al., 2020).
Preparation of curcumin tetrahydro-indazole analogs (3a-d)
The preparation of 3a with a yield of 61.0% has been reported previously (Hariyanti et al., 2020). Preparation of compounds 3b-d was carried out according to the procedures of compound 3a by reacting 1b that replaced 1a with p-methoxy-benzaldehyde, vanillin, or 3,4-dimethoxy-benzaldehyde (2a-c).
4-([(7E)-3–(3,4–dimethoxyphenyl)–4,5,6,7–tetrahydro–3aH–indazol-7-ylidene]methyl)-2-methoxyphenol (3b)
Pale yellow powder, yield 35.2%, and m.p. 240°C–242°C. Fourier Transform Infrared (FT-IR), υ/cm−1: 3,217 (OH), 3,059 (C-H Ar stretch ), 2,956 (C-H Al stretch), 1,582 (C=N), 1,519 (C=C ring Ar stretch), 1,460 C-H Al bend), 1,253 (C-O-Ar asymmetrical stretch), and 1,162 (C-O-Ar symmetrical stretch ). 1H-NMR, δ/ppm: 7.24 (d, 1H, J 3 Hz), 6.94 (d,02H, J 8 Hz), 7.17 (dd,01H, J 8 Hz), 6.89 (dd,02H, J 8 Hz), 3.85 (t,01H), 3.91 (d, 6H), 3.92 (s, 3H), 2.81 (m,04H), 1.97 (m,02H). 13C-NMR, δ/ppm: 149.35 (1C), 149.21 (1C), 148.64 (1C), 148.36 (1C), 145.53 (1C), 144.13 (1C); 129.9 and 114.8 (C=Cethylenic), 1,122.4 (2CAr); 123.9, 119.9, 112.8,0,111.1,0110.9, and 110.2 (1CAr, respectively); 56.0, 55.1, and 55.06 (1CO-Me, respectively);227.2, 31.2, 24.2, and 22.2 (1CAl, respectively). HR-MS:-m/z 393.18003 [M+H]+; calculated for C23H24N2O4 = 392.17361 [M]; Error = - 2.3 ppm.
(7E)–3–(3,4–dimethoxyphenyl)–7–(4–methoxybenzylidene)–4,5,6,7–tetrahydro–3aH–indazole (3c)
Pale yellow powder, yield 74.3%, and m.p. 244°C–246°C. FT-IR, υ/cm−1: 3,020 (C-H Ar stretch), 2,920 (C-H Al stretch), 1,561 (C=N), 1,519 (C=C ring Ar stretch), 1,460 (C-H Al bend), 1,261 (C-O-Ar asymmetrical stretch), and 1,136 (C-O-Ar symmetrical stretch). 1H-NMR, δ/ppm: 7.23 (d, 1H, J 1.5 Hz), 6.89 (d, 2H, J 9 Hz), 7.17 (dd, 1H, J 8 Hz), 6.97 (s, 1H), 6.94 (dd, 1H, J 8 Hz), 6.87 (d, 1H, J 8.5 Hz),03.92 (s, 3H),03.91 (d,06H),02.81 (m, 4H), 1.88 (m,02H). 13C-NMR, δ/ppm: 149.22 (1C), 148.65 (1C), 148.29 (1C), 145.73 (1C), 144.02 (1C); 129.6 and 122.4 (C=Cethylenic); 126.2, 123.9, 119.9, 114.8, 112.8,0111.2,0111.1, 110.95, and 110.92 (1CAr, respectively); 56.1, 56.05, and 55.0 (1CO-Me, respectively); 27.2, 31.2,024.7, and 22.3 (1CAl, respectively). HR-MS:-m/z 409.21152 [M+CH3OH+H]+; calculated for C23H24N2O3= 376.17896 [M]; Error = 0.3 ppm.
(7E)–3–(3,4–dimethoxyphenyl)–7–(3,4–dimethoxybenzylidene)–4,5,6,7–tetrahydro–3aH–indazole (3d)
Pale yellow powder, yield 56.1%, and m.p. 236°C–238°C. FT-IR, υ/cm−1: 3,020 (C-H Ar stretch), 2,947 (C-H Al stretch), 1,591 (C=N), 1,514 (C=C ring Ar stretch), 1,450 (C-H Al bend), 1,255 (C-O-Ar asymmetrical stretch), and 1,142 (C-O-Ar symmetrical stretch). 1H-NMR, δ/ppm: 6.88-(s, 2H), 7.19 (dd,01H, J 8 Hz), 6.91 (s, 2H), 6.87 (s,01H), 6.94 (dd, 2H, J 8 Hz), 3.93 (s, 3H),03.92 (s, 3H), 3.91 (s, 3H), 3.89 (s, 3H), 2.81 (m, 4H), 1.88 (m, 2H). 13C-NMR, δ/ppm: 149.27 (1C), 148.94 (1C), 148.71 (1C), 148.16 (1C),0129.85 (1C),0128.0 (1C); 122.12 and 121.3 (C=Cethylenic); 119.42, 109.92, and 111.04 (2CAr, respectively); 112.66 and 111.28 (1CAr, respectively); 56.08 and 56.04 (2CO-Me, respectively); 31.15, 27.24,024.81, and 22.38 (1CAl, respectively). HR-MS :-m/z 407.19619 [M+H]+; calculated for C24H26N2O3 = 406.18926 [M]; Error = - 0.7 ppm.
Preparation of curcumin hexahydro-indazole analogs (5a–b)
Preparation of 5a-b was carried out by mixing 10 mmol of the synthesized cyclovalone (4a) for 5a and its p-hydroxy analog (4b) for 5b, with 100 mmol hydrazine monohydrate in 10 ml of glacial acetic acid, refluxed at 120°C for 3 hours until completed reaction, added on to crushed ice, filtered off till the suspension was obtained, and washed using cold water to afford the solid product. Recrystallization was done from a suitable solvent to provide the pure compound 5a–b.
1-[(7E)-3-–(4-hydroxy,3–methoxyphenyl)–7–(4-hydroxy,3–methoxybenzylidene)-3,3a,4,5,6,7-hexahydro–2H–indazol-2-yl]-ethan-1-one (5a)
Pale yellow powder, yield 23.7%, and m.p. 105°C–107°C. FT-IR, υ/cm−1: 3,200–3,525 (OH), 3,059 (C-H Ar stretch), 2,956 (C-H Al stretch), 1,635 (C=O), 1,599 (C=N), 1,518 (C=C ring Ar stretch), 1,450 (C-H Al bend), 1,273 (C-O-Ar asymmetrical stretch), and 1,150 (C-O-Ar symmetrical stretch). 1H-NMR, δ/ppm: 7.14 (s, 1H), 6.92 (d, 2H, J 2-Hz), 6.75 (d,01H, J 2 Hz), 6.86 (s,01H), 6.78 (dd,01H, J 8Hz), 6.88 (d, 1H, J 8Hz), 3.90 (d, 6H), 2.19 (s, 3H), 4.83 (d, 1H), 3.06 (d, 1H), 2.39 (s, 2H), 2.32 (m, 1H), 2.43 (m, 1H), 2.96 (m, 1H), 1.94 (m, 2H). 13C-NMR, δ/ppm: 170.64 (1CC=O), 159.08 (1CC=N);0146.82,0146.38, 145.61, 145.06 (1CAr-O, respectively); 0128.98 and 128.19 (C=Cethylenic); 134.11, 128.64, 123.47, 118.63, 114.86, 114.46, 112.38, and 108.48 (1CAr, respectively); 67.99 (1CC-N); 57.43 and 56.08 (1CO-Me, respectively); 38.96, 30.15, 29.06,024.49, and 22,49 (1CAl, respectively). HR-MS:-m/z 423.19132 [M+H]+; calculated for C24H26N2O5 = 422.18417 [M]; Error = - 0.2 ppm.
1-[(7E)-3-(4-hydroxyphenyl)–7–(4-hydroxybenzylidene)-3,3a,4,5,6,7–hexahydro–2H–indazol- 2-yl]-ethan-1-one (5b)
Pale yellow powder, yield 42.2%, and m.p. 146°C–148°C. FT-IR, υ/cm−1: 3,200–3,500 (OH), 3,012 (C-H Ar stretch), 2,943 (C-H Al stretch), 1,647 (C=O), 1,579 (C=N), 1,512 (C=C ring Ar stretch), 1,450 (C-H Al bend), and 1,263 (CAr-O stretch). 1H-NMR, δ/ppm: 6.74 (m,01H, J 2 Hz), 7.19 (dd, 2H, J 8 Hz), 6.91 (s,01H), 6.84 (dd,01H, J 6 Hz), 7.05 (m,01H, J 8 Hz), 6.78 (dd, 2H, J 8 Hz), 2.13 (s, 3H), 4.07 (m,01H), 3.06 (d,01H), 2.72 (m,02H), 2.0 (s,01H), 1.8 (m,01H), 1.21 (m, 2H). 13C-NMR, δ/ppm: 170.6 (1CC=O), 159.97 (1CC=N); 156.63 and 155.55 (1CAr-O, respectively); 0114.16 and0115.20 (C=Cethylenic); 131.36, 130.71, 128.88, 128.12, 126.99, 121.35, 115.70, 115. 63, 115. 28 and 115.20 (1CAr, respectively); 67.43 (1CC-N); 30.05, 29.76, 29.13, 27.20 and 24.67 (1CAl, respectively). HR-MS:-m/z 363.17051 [M+H]+; calculated for C23H22N2O3 = 362.16304 [M]; Error = +0.8 ppm.
In vitro cytotoxicity
The prepared compounds were tested for cytotoxicity against MCF-7, HeLa, WiDr, and vero cell lines (American Type Culture Collection ([ATCC) HTB-22, ATCC CCL-2, ATCC CCL-218, and ATCC CCL-81] using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) assay procedure (ATCC, 2020). Fetal bovine serum (5%), penicillin (100 U/ml), and streptomycin (100 µg/ml) were added to the 96-well plates containing cultivated cells in suitable growth media (Dulbecco's Modified Eagle's medium for WiDr and vero and Roswell Park Memorial Institute 1640 medium for MCF-7 and HeLa) and incubated for 24 hours until the culture reached 60% confluence. After replacing the growth media with new media, a series of concentrations (3.125–200 μg/ml) of test compounds were added. The cells were reincubated for 48 hours (the growth media were replaced every day with new media). After that, a 10 µl MTT solution was added and incubated for 4 hours; the media were discarded and the formazan formed in 100 µl ethanol was dissolved. Finally, the dissolved formazan was measured using an Enzyme-Linked Immunosorbent Assay reader at λ 595 nm (Bahuguna et al., 2017). The IC50 values were determined from the curve of concentrations versus inhibitions (%). GraphPad Prism 8 v. 8.02 (www.graphpad.com) was used for analysis.
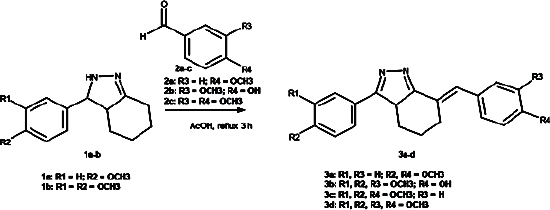 | Figure 1. Preparation reaction of compounds 3a-d. [Click here to view] |
RESULTS AND DISCUSSION
Chemistry
Compounds 3b-d were prepared by condensation 1b-and-benzaldehyde derivatives-2a-c in a yield of 35.2%, 56.1%, and 74.3% (Fig 1), while compounds 5a-b were prepared by condensation symmetrical bis-benzylidene-cyclohexanone 4a-b and hydrazine monohydrate in a yield of 23.7% and 42.2% (Fig 2). The reactions were carried out in glacial acetic acid and reflux temperature to afford novel products 4,5,6,7-tetrahydro-3aH-indazole (3b-d) and 2-acetylated-3,3a,4,5,6,7-hexahydro-2H-indazole (5a-b). The high temperature applied in the reaction most likely lowered the stability of the starting materials (Weerawatanakorn et al., 2015), causing low yields. The application of mild conditions resulted in a higher yield; however, the products were different from those above (Bayomi et al., 2015; Nuriev et al., 2016; Raut et al., 2020).
The prepared compound structures were elucidated based on spectral analysis. No sharp peak at 3,290–3,300 cm−1 appeared in the FT-IR spectra, indicating the disappearance of the amine group. The bands at 3,012–3,059 and 2,920–2,956 cm−1 confirmed the presence of C-H aromatic and aliphatic bonds. The C=N azole, C=C aromatic ring, and C-O-C ether bonds were observed at 1,732–1,734, 1,447–1,665, and 1,140–1,265 cm−1, respectively. Broad peaks at 3,217 and 3,200–3,500 cm−1 indicated a hydroxyl group’s presence in the 3b and 5a-b compounds, while a strong peak at 1,635–1,647 cm−1 confirmed the C=O of acetamide in the 5a and 5b compounds. In the 1H-NMR spectra, 7–9 protons for the two aromatic rings and one ethenyl chain appeared at 6–7 ppm, and a typical proton OCH3 group appeared at around 3.8 ppm. Structural analysis was also supported by 13C-NMR and the mass spectral data, which confirmed the suitability to the targeted compounds (MarvinSketch 20.8.0, 2020; Silverstein et al., 2005).
In vitro cytotoxicity
The prepared compounds (3a-d and 5a-b) were evaluated for their cytotoxicity against four cell lines (MCF-7, HeLa, WiDr, and vero) using an MTT assay procedure. Curcumin, tamoxifen, and doxorubicin were used as a comparable compound and positive control. Tamoxifen is a drug commonly used in breast cancer treatment, while doxorubicin is most useful for treating broad cancers such as leukemia, neuroblastoma, and ovary, lung, and breast cancer (Thorn et al., 2011).
The results indicated that the prepared compounds had low to medium cytotoxic activity (Table 1 and Fig. 3). The IC50 values against MCF-7 cells were between 45.97 and 86.24 µM. Compound 3b had the highest cytotoxicity against MCF-7, but its activity was lower than curcumin. In contrast, tamoxifen and doxorubicin exhibited high cytotoxicity against MCF-7. The IC50 values against HeLa cells were between 46.36 and 100 µM. Compound 3d had the highest cytotoxicity against HeLa cells. The cytotoxicity of 3d was higher than that of curcumin but lower than tamoxifen and doxorubicin. The IC50 values against WiDr cells were between 27.20 and 58.19 μM. These results indicated that the synthesized compounds were more cytotoxic against WiDr than against HeLa and MCF-7 cells. -Compound 3b had the highest cytotoxicity against WiDr cells. This activity was better than curcumin and tamoxifen but lower than doxorubicin.
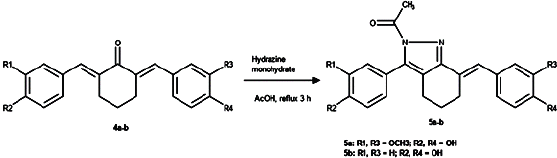 | Figure 2. Preparation reaction of compounds 5a-b. [Click here to view] |
 | Figure 3. Cytotoxic activity (IC50, µM) values for the prepared compounds, curcumin, tamoxifen, and doxorubicin on MCF-7, HeLa, WiDr, and vero cells, respectively. [Click here to view] |
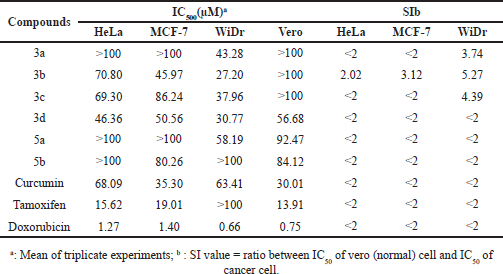 | Table 1. IC50 and SI values of the prepared compounds, curcumin, tamoxifen, and doxorubicin against MCF-7, HeLa, WiDr, and vero cell lines. [Click here to view] |
The cytotoxicity of compounds 3b, 3c, and 3d containing three and four methoxy groups was higher than compounds 3a, 5a, and 5b containing less than three methoxy groups. The results were in line with the finding previously reported in asymmetrical analogs of curcumin and 4-amino chalcone derivatives that the methoxy group’s number and position influence cytotoxic activity (Prasetyaningrum et al., 2018; Novilla et al., 2019).
The IC50 values against vero cells were between 56.68 and >100 µM. These data indicated that most synthesized compounds had low toxicity against normal cells (Burger et al., 2004; Schmitz et al., 1993). Compound 3b showed high selectivity against MCF-7, HeLa, and WiDr cells [selectivity index, (SI) = 3.12, 2.02, and 5.27]. In contrast, compounds 3a and 3c only showed selectivity against WiDr cells (SI = 3.74 and 4.39). The selectivity of these compounds was higher than curcumin and the positive controls (tamoxifen and doxorubicin). The compound with an SI less than two indicates general toxicity. The higher the SI, the more selective the compound (Burger et al., 2004; Badisa et al., 2009; Kurnia et al., 2019). The IC50 value of curcumin against MCF-7 cells was 35.03 μM, whereas in a previous study it was 8.62 μM (Li et al., 2015). This difference may be due to the different methods used for the testing or different laboratory conditions. The resistance factor also affects the sensitivity of the drug to cells.
CONCLUSION
A series of six curcumin indazole analogs have been prepared. All the compounds showed low to moderate cytotoxicity against MCF-7, HeLa, and WiDr cells. Compounds 3b exhibited the greatest cytotoxicity, especially against WiDr cells with excellent selectivity. The compound should be further developed as a cytotoxic agent for colorectal adenocarcinoma.
ACKNOWLEDGMENTS
The authors are grateful to the Chemical Research Center, LIPI, Tangerang, Indonesia, for NMR and MS measurements.
CONFLICT OF INTEREST
The authors confirm no conflicts of interest between the authors.
FUNDING
This research and APC were supported by the Ministry of Research and Technology/ BRIN, RI, 2020 (Assignment Agreement No. NKB-0373/UN2.RST/HKP.05.00/2020).
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included within this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
American Cancer Society. Cancer facts & figures. American Cancer Society, pp 1–44, 2020. Available via https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2020.html (Accessed 1 December 2020).
Badisa RB, Darling-Reed SF, Joseph P, Cooperwood JS, Latinwo LM, Goodman CB. Selective cytotoxic activities of two novel synthetic drugs on human breast carcinoma MCF-7 cells. Anticancer Res, 2009; 29:2993–6.
Bahuguna A, Khan I, Bajpai VK, Kang SC. MTT assay to evaluate cytotoxic potential of a drug. Bangladesh J Pharmacol, 2017; 12:115–8; doi:10.3329/bjp.v12i2.30892 CrossRef
Bayomi SM, El-kashef HA, El-ashmawy MB, Nasr MNA, El-sherbeny MA, Abdel-aziz NI, El-Sayed MA, Suddek GM, El-Messer SM, Ghaly MA. Synthesis and biological evaluation of new curcumin analogues as antioxidant and antitumor agents: molecular modelling study. Eur J Med Chem, 2015; 101:584–94; doi:10.1016/j.ejmech.2015.07.014 CrossRef
Bayomi SM, El-Kashef HA, El-Ashmawy MB, Nasr NA, El-Sherbeny MA, Badria A. Synthesis and biological evaluation of new curcumin derivatives as antioxidant and antitumor agents. Med Chem Res, 2013; 22:1147–62; doi:10.1007/s00044-012-0116-9 CrossRef
Burger AM, Fiebig HH. Preclinical screening for new anticancer agents. In: Figg WD, McLeod HL (eds.). Handbook of anticancer pharmacokinetics and pharmacodynamics, cancer drug discovery and development, Humana Press Inc, Totowa, NJ, pp 36–7, 2004; doi:10.1007/978-1-59259-734-5_2 CrossRef
Gaikwad DD, Warad KD, Chapolikar AD, Devkate CG, Tayade AP, Pawar RP, Domb AJ. Synthesis of indazole motifs and their medicinal importance: an overview. Eur J Med Chem, 2015; 90:707–31; doi:10.1016/j.ejmech.2014.11.029 CrossRef
GraphPad Software, Inc. Available via www.graphpad.com (Accessed 17 November 2020).
Hariyanti H, Kusmardi K, Yanuar A, Hayun H. Ligand based pharmacophore modeling, virtual screening, and molecular docking studies of asymmetrical hexahydro-2H-indazole analogs of curcumin (AIACs) to discover novel estrogen receptors alpha (ERα) inhibitor. Indones J Chem, 2021; 21(1):137–47; doi:10.22146/ijc.54745 CrossRef
Hariyanti H, Yanuar A, Kusmardi K, Hayun H. (7E)–3–(4–Methoxyphenyl)–7–[(4–methoxyphenyl) methylidene]–4,5,6,7–tetrahydro–3aH–indazole. Molbank, 2020; 2020:M1162; doi:10.3390/M1162 CrossRef
Hayun H, Jatmika C, Purwati EM, Salim S, Kurniawan R, Chandra EG, Fajriawan AA, Nareswara AD. Synthesis and free radical-scavenging activities of di-mannich bases of cyclovalone derivatives. Orient J Chem, 2017; 33(6):2742–57; doi:10.13005/ojc/330607 CrossRef
Kurnia A, Saputri FC, Hayun H. Synthesis and anticancer potential of aminomethyl derivatives of methyl-substituted asymmetrical curcumin mono-carbonyl. J Appl Pharm Sci, 2019; 9(08):018–24; doi.10.7324/JAPS.2019.90803 CrossRef
Li Q, Chen J, Luo S, Xu J, Huang Q, Liu T. Synthesis and assessment of the antioxidant and antitumor properties of asymmetric curcumin analogues. Eur J Med Chem, 2015; 93:461–9; doi:10.1016/j.ejmech.2015.02.005 CrossRef
MarvinSketch 20.8.0. ChemAxon Ltd., 1998–2020. Available via http://www.chemaxon.com
Minu M, Thangadurai A, Wakode SR, Agrawal SS, Narasimhan B. Synthesis, antimicrobial activity and QSAR studies of new 2,3-disubstituted-3,3a,4,5,6,7-hexahydro-2H-indazoles. Bioorg Med Chem Let, 2009; 19(11):2960–4; doi:10.1016/j.bmcl.2009.04.052 CrossRef
MTT cell proliferation assay. Available via https://www.atcc.org/~/media/DA5285A1F52C414E864C966FD78C9A79.ashx (Accessed 28 November 2020).
Nuriev VN, Vatsadze IA, Sviridenkova NV, Vatsadze SZ. Synthesis of 3,7 disubstituted hexahydro and tetrahydro-2H-indazoles from cross-conjugated dienones. Russ J Org Chem, 2016; 52:389–96; doi:10.1134/S1070428016030167 CrossRef
Novilla A, Mustofa M, Astuti I, Jumina J, Suwito H. Cytotoxic activity of methoxy-4-amino chalcone derivatives against leukemia cell lines. Mol Cell Biomed Sci, 2019; 3(1):34–41; doi:10.21705/mcbs.v3i1.44 CrossRef
Plescia S, Raffa D, Plescia F, Casula G. Synthesis and biological evaluation of new indazole derivatives. ARKIVOC, 2010; (x):163–77; doi:10.3998/ark.5550190.0011.a14 CrossRef
Prasetyaningrum PW, Bahtiar A, Hayun H. Synthesis and cytotoxicity evaluation of novel asymmetrical mono-carbonyl analogs of curcumin. Sci Pharm, 2018; 86(2):25; doi:10.3390/scipharm86020025 CrossRef
Rahmawati N, Hariyanti H, Saputri FC, Hayun H. Synthesis and preliminary in vitro anti-inflammatory evaluation of mannich bases derivatives of 4-methoxy-substituted of asymmetrical cyclovalone analogs. Indones J Pharm, 2020; 31(1):35–41; doi:10.13005/ojc/330607. CrossRef
Raut S, Dhotre B, Tidke A, Pathan MA. An operationally simple and efficient synthesis of 7-benzylidene-substitutedphenyl- 3,3a,4,5,6,7-hexahydro-2H-indazole by grinding method. Curr Org Syn, 2020; 17(4):313–21; doi: 10.2174/1570179417666200406142118 CrossRef
Schmitz FJ, Bourden BF, Toth SI. Antitumor and cytotoxic compounds from marine organisms. In: Attaway DH, Zaborsky OR (eds.). Marine biotechnology, pharmaceutical and bioactive natural products, Plenum Press, New York, NY, 198 p, vol. 1, 1993.
Shrivastava A, Chakraborty AK, Upmanyu N, Singh A. Recent progress in chemistry and biology of indazole and its derivatives: a brief review. Austin J Anal Pharm Chem, 2016; 3(4):1076. Available via https://austinpublishinggroup.com/analytical-pharmaceutical-chemistry/fulltext/ajapc-v3-id1076.php
Silverstein RM, Webster FX, Kiemle DJ. 2005. Spectrometric identi?cation of organic compounds. 7th ed, John Wiley & Sons, Inc, New York, NY.
Thangadurai A, Minu M, Wakode S, Agarwal S, Narasimhan B. Indazole: a medicinally important heterocyclic moiety. Med Chem Res, 2012; 21:1509–23; doi:10.1007/s00044-011-9631-3 CrossRef
Thirupalu RM, Hanuman RV, Chenna KRR, Krishna V, Rami RYV. Synthesis and molecular docking studies of new substituted indazole derivatives for anti-breast cancer activity. Der Pharm Chem, 2014; 6(6):411–7; Available via https://1library.net/document/zp033loq-synthesis-molecular-docking-studies-substituted-indazole-derivatives-activity.html
Thorn CF, Oshiro C, Marsh S, Hernandez-Boussard T, McLeod H, Klein TE, Altman RB. Doxorubicin pathways: pharmacodynamics and adverse effects. Pharmacogenet Genom, 2011; 21(7):440–6; doi:10.1097/FPC.0b013e32833ffb56 CrossRef
Weerawatanakorn M, Wu JC, Pan MH, Ho CT. Reactivity and stability of selected ?avor compounds. J Food Drug Anal, 2015; 23(2):176–90; doi:10.1016/j.jfda.2015.02.001 CrossRef
Zhang SG, Liang CG, Zhang WH. Recent advances in indazole-containing derivatives: synthesis and biological perspectives. Molecules, 2018; 23:2783; doi:10.3390/molecules23112783 CrossRef