INTRODUCTION
Amomum krervanh Pierre (Zingiberaceae) is a tropical plant that is widely distributed in Southeast Asia. It is used globally for spices and is commonly applied as folk medicine to treat stomach disorders (Yin et al., 2013a). The chemical composition of this plant has been reported (Wu et al., 2006; Yin et al., 2013a, 2013b; Zeng et al., 2012) and it has also been indicated that plants of the genus Amomum exhibit antioxidant (Teresita et al., 2000), antimicrobial (Diao et al., 2014; Kwon et al., 2003; Malti et al., 2007; Moon et al., 2004), anti-inflammatory (Choi et al., 2018; Lee et al., 2008; Mathew et al., 2003), and antimalarial activities (Kamchonwongpaisan et al., 1995).
Recently, in studies on the endophytic actinomycetes, we isolated strain W08 from the pseudostem tissue of Amomum krervanh Pierre. It has antibacterial activity against mostly Gram-positive bacteria. Herein, we report the cytotoxicity of its crude extract against two normal cell lines [murine epithelial cells (L929) and African green monkey kidney cells (Vero)] and three cancer cell lines [human breast carcinoma cells (MCF-7), human cervical carcinoma cells (HeLa), and human hepatocellular carcinoma cells (HepG2)] using a (3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide) (MTT) colorimetric assay. We also identified this strain, purified the major compounds, and also elucidated their structures.
MATERIALS AND METHODS
Isolation, cultivation, and antibacterial screening of endophytic actinomycetes
Various tissues of Amomum krervanh Pierre were used to isolate the endophytic actinomycetes as described in a previous study (Taechowisan et al., 2017). Ten actinomycete isolates were obtained and tested for their ability to produce antibacterial substances against Bacillus cereus ATCC 7064, Bacillus subtilis ATCC 6633, Escherichia coli ATCC 25922, methicillin-resistant Staphylococcus aureus Sp6, Pseudomonas aeruginosa ATCC 28753, and S. aureus ATCC 25923 using an overlay assay on ISP-2 agar plates, as previously described (Wu et al., 2015) with some modifications. Antibacterial screening of actinomycetes was carried out as described in a previous study (Taechowisan et al., 2017). Among the 10 isolates of actinomycetes, the results showed that the best production of antibacterial substances was achieved by isolate W08. This isolate was identified according to the methods of Taechowisan et al. (2003)and Taechowisan et al. (2017). The isolate W08 was grown on ISP-2 agar at 30°C for 14 days, and the culture was extracted with ethyl acetate as described in a previous study (Taechowisan et al., 2017).
Minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC)
The MICs of the crude extract were carried out by the National Committee for Clinical Laboratory Standards microbroth dilution methods (National Committee for Clinical Laboratory Standards, 2000). The crude extract was initially dissolved in dimethyl sulfoxide (DMSO). The MIC and MBC were carried out as described in a previous study (Taechowisan et al., 2017).
MTT assay for cytotoxicity activity (IC50)
The normal cell lines [murine epithelial cells (L929) and African green monkey kidney cells (Vero)] and three cancer cell lines [HeLa, human hepatocellular carcinoma cells (HepG2), and MCF-7] were used to assess the IC50 of the crude extract using MTT assay as described in a previous study (Taechowisan et al., 2017).
Purification and structural elucidation of major components
Ethyl acetate extract (10.75 g) was fractionated by silica gel 60 column chromatography and eluted with a mixture of petroleum ether–ethyl acetate with increasing polarity. Fractions eluted with 3.5% and then 8% ethyl acetate in petroleum ether were purified by thin-layer chromatography (TLC) (solvent: petroleum ether–ethyl acetate, 8.5:1.5 and 4:1) to give compounds 1 and 2, respectively. The purified compounds were then subjected to NMR spectroscopy.
RESULTS AND DISCUSSION
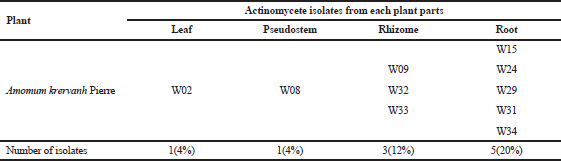
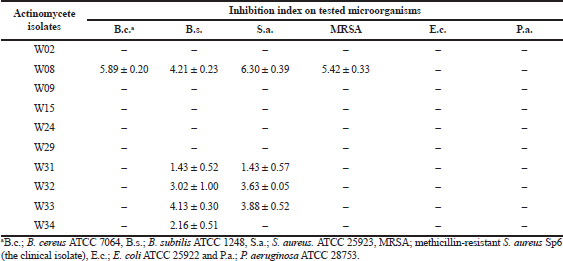
A total of 100 samples each of leaf, pseudostem, rhizome, and root tissues of Amomum krervanh Pierre were examined. The root tissue was the site where actinomycetes were most commonly isolated, where a total of 20% of actinomycetes were found (Table 1), which matches our previous report (Taechowisan et al., 2003). The roots thus present a good habitat for these endophytic actinomycetes. This may be related to the abundance of actinomycetes within the rhizosphere microbial flora (Sardi et al., 1992), enabling easier infection of host plants. The results showed that, against the tested microorganisms, isolate W08 showed promising activities (Table 2). A large number of endophytic actinomycete isolates have no potential antibacterial activity against the tested bacteria. As stated in several reports, endophytic actinomycete activity influences the metabolic products of actinomycetes acting on plant growth and physiology (Hasegawa et al., 2006; Igarashi et al., 2002; Manulis et al., 1994; Meguro et al., 2006; Mishra et al., 1987). Thus, most of them did not produce antimicrobial metabolic products.
| Table 1. Isolated actinomycetes from different parts of Amomum krervanh Pierre. |
| Table 2. Screening for antibacterial activity of actinomycetes using an overlay assay. |
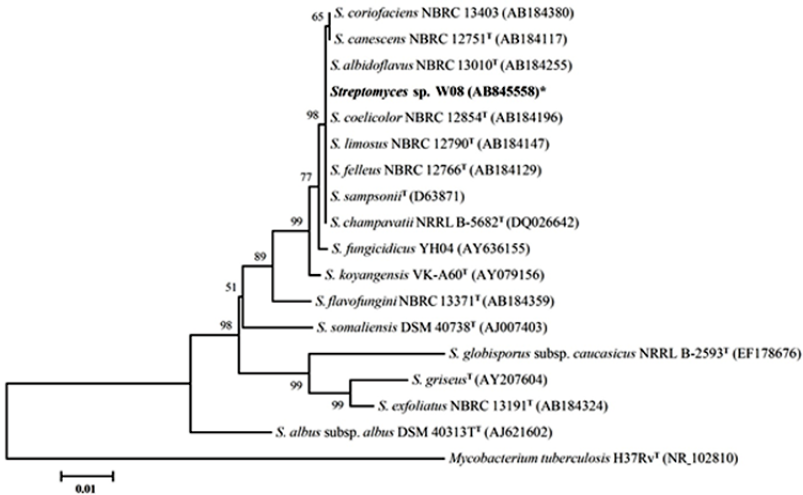
Based on the results of morphological observation, isolate W08 formed extensive substrate mycelia and abundant aerial mycelia on agar medium. It produced aerial mycelia that differentiated into long spore chains. These spore chains were straight to flexuous (rectiflexibiles) with oval and smooth surface spores (Figure. 1). Its substrate mycelia were grayish-green to dark olive-brown and the aerial mycelia were greenish-white to light greenish-grey. Owing to features including the presence of LL-type diaminopimelic acid in the whole-cell extracts, isolate W08 was identified as Streptomyces. The 16S rDNA sequence was determined for isolate W08 (1,473 bp). The BLAST search results and the phylogenetic tree are presented in Table 3 and Figure 2, respectively. These results showed that isolate W08 has high levels of sequence similarity to Streptomyces coelicolor NBRC 12854 (accession number: AB184196) and Streptomyces felleus NBRC 12766 (accession number: AB184129). The 16S rDNA analysis showed that isolate W08 is phylogenetically also closely related to these strains. The 16S rDNA sequence data reported in this paper are available in GenBank under accession number AB845558.
The antibacterial activity of the crude extract from Streptomyces sp. W08 culture is shown in Table 4. The crude extract inhibited the tested bacteria, with MICs and MBCs of 32–128 µg/ml and 256 to >512 µg/ml, respectively. However, no activity against Gram-negative bacteria was exhibited by this extract, which was due to the different composition of bacterial cell walls. The mechanisms of action of the crude extract may involve antimicrobial effects, having different activity against Gram-negative and Gram-positive bacteria.
| Figure 1. The morphological characteristics of Streptomyces sp. W08. The colony appearance [surface (a) and reverse (b)] of Streptomyces sp. W08 after 21 days of growth on ISP-2 agar at 30°C incubation. (c) a light micrograph and (d) a scanning electron micrograph; bar = 2 μm. |
The crude extract showed weak cytotoxic activity against L929 and Vero cells, with IC50 values of 453.22 and >512.00 µg/ml, respectively (Table 5). The most powerful cytotoxicity of the crude extract was observed in HeLa and MCF-7 cells, with IC50 values of 78.45 and 106.50 µg/ml, respectively, while the IC50 value against HepG2 cells was 425.86 µg/ml. These data are interesting as they suggest that this crude extract is more toxic to some cancer cells than to normal cells, implying its potential as an anticancer agent. Although this preliminary anticancer study demonstrated that the crude extract exhibited an anticancer effect, more detailed investigation is required to isolate the bioactive compounds from the crude extract.
Among our observations of the cytotoxicity toward cancer cells, it was particularly interesting that the crude extract exhibited greater toxicity against HeLa and MCF-7 cells than against L929 and Vero cells. In this context, the therapeutic index (TI) is an important parameter to select samples for developing drugs. This value is the ratio of the concentration of the crude extract at which 50% of the normal cell death to that of the crude extract at which 50% cancer cell death occurred. Further investigation is considered to be warranted if a TI value of ≥4 is identified. The present study found TI values for this crude extract of ≥4 for HeLa and MCF-7 cells. This extract is thus promising for further investigation as an anticancer agent.
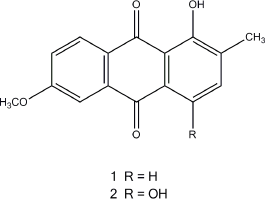
Silica gel column chromatography and TLC with petroleum ether–ethyl acetate as the mobile phase resulted in the isolation of two major compounds, 1-hydroxy-2-methyl-6-methoxyanthraquinone (1) and 6-methoxy-2-methylquinizarin (2), TOFMS m/z: 291.2533 (calcd for C16H12O4Na: 291.2532) and 307.2527 (calcd for C16H12O5Na: 307.2525), respectively (Figure. 3). Their 1H-NMR and 13C-NMR spectral data were identical with those of 1-hydroxy-2-methyl-6-methoxyanthraquinone and 6-methoxy-2-methylquinizarin previously reported (El-Lakany et al., 2004). According to data from the literature, anthraquinone compounds have been reported on antimicrobial activity (El-Lakany et al., 2004; Mbaveng et al., 2008).
| Table 3. 16S rDNA similarity values between Streptomyces sp. W08 and representatives of the genus Streptomyces. |
| Figure 2. Neighbor-joining phylogenetic tree of isolate W08, including the closely related type strain base on 16SrDNA gene sequences which were retrieved from GenBank. Accession numbers appear in parentheses. Bootstrap (1,000 replicates) values are given in percentage. Bar, 0.01 substitutions per site. |
| Table 4. MIC and MBC of the crude extract from Streptomyces sp. W08 culture. |
| Table 5. IC50 and TI of the crude extract on various cell lines. |
| Figure 3. Chemical structures of 1-hydroxy-2-methyl-6-methoxyanthraquinone (1) and 6-methoxy-2-methylquinizarin (2). |
CONCLUSION
In summary, crude extract from the culture of Streptomyces sp. W08 showed antibacterial activity against Gram-positive bacteria. It also exhibited potent cytotoxic effects on HeLa and MCF-7 cells, while exhibiting low cytotoxicity against normal cells (L929 and Vero cells). These results indicate that this crude extract may be a potential therapeutic candidate for treating some bacterial infections and cancers.
ACKNOWLEDGMENT
This work was supported by the Faculty of Science, Silpakorn University, Nakhon Pathom, Thailand.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Choi CW, Shin JY, Seo C, Hong SS, Ahn EK, Jung YH, Oh JS. In vitro anti-inflammatory activity of the components of Amomum tsao-ko in murine macrophage RAW 264.7 cells. Afr J Tradit Complement Altern Med, 2018; 15:26–34. CrossRef
Diao WR, Zhang LL, Feng SS, Xu JG. Chemical composition, antibacterial activity, and mechanism of action of the essential oil from Amomum kravanh. J Food Prot, 2014; 77:1740–6. CrossRef
El-Lakany AM, Aboul-Ela MA, Abdel-Kader MS, Badr JM, Sabri NN, Goher Y. Anthraquinones with antibacterial activities from Crucianella maritima L. growing in Egypt. Nat Prod Sci, 2004; 10:63–8.
Hasegawa S, Meguro A, Shimizu M, Nishimura T, Kunoh H. Endophytic actinomycetes and their interactions with host plants. Actinomycetologica, 2006; 20:72–81. CrossRef
Igarashi Y, Iida T, Yoshida R, Furumai T. Pteridic acids A and B, novel plant growth promoters with auxin-like activity from Streptomyces hygroscopicus TP-A0451. J Antibiot, 2002; 55:764–7. CrossRef
Kamchonwongpaisan S, Nilanonta C, Tarnchompoo B, Thebtaranonth C, Thebtaranonth Y, Yuthavong Y, Kongsaeree P, Clardy J. An antimalarial peroxide from Amomum krervanh Pierre. Tetrahedron Lett, 1995; 36:1821–4. CrossRef
Kwon KB, Kim JH, Lee YR, Lee HY, Jeong YJ, Rho HW, Ryu DG, Park JW, Park BH. Amomum xanthoides extract prevents cytokine-induced cell death of RINm5F cells through the inhibition of nitric oxide formation. Life Sci, 2003; 73:181–91. CrossRef
Lee JY, Kim SH, Sung SH, Kim YC. Inhibitory constituents of lipopolysaccharide -induced nitric oxide production in BV2 microglia isolated from Amomum tsao-ko. Planta Med, 2008; 74:867–9. CrossRef
Malti JE, Mountassif D, Amarouch H. Antimicrobial activity of Elettaria cardamomum: toxicity, biochemical and histological studies. Food Chem, 2007; 104:1560–8. CrossRef
Manulis S, Shafrir H, Epetein E, Lichter A, Barash I. Biosynthesis of indole-3-acetic acid via indole-3-acetamide pathway in Streptomyces spp. Microbiology, 1994; 140:1045–50. CrossRef
Mathew J, Shiburaj S, George V. Antimicrobial activity of Amomum cannicarpum. Fitoterapia, 2003; 74:476–8. CrossRef
Mbaveng AT, Kuete V, Nguemeving JR, Penlap BV, Nkengfack AE, Meyer JJM, Lall N, Krohn K. Antimicrobial activity of the extracts and compounds obtained from Vismia guineensis (Guttiferae). Asian J Tradit Med, 2008; 3:211–23.
Meguro A, Ohmura Y, Hasegawa S, Shimizu M, Nishimura T, Kunoh H. An endophytic actinomycete, Streptomyces sp. MBR-52, that accelerates emergence and elongation of plant adventitious roots. Actinomycetologica, 2006; 20:1–9. CrossRef
Mishra SK, Taft WH, Putnam AR, Ries SK. Plant growth regulatory metabolites from novel actinomycetes. J Plant Growth Regul, 1987; 6:75–84. CrossRef
Moon SS, Lee JY, Cho SC. Isotsaokoin, an antifungal agent from Amomum tsao-ko. J Nat Prod, 2004; 67:889–91. CrossRef
National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard M7-A5. National Committee for Clinical Laboratory Standards, Wayne, PA, 2000.
Sardi P, Saracchi M, Quaroni S, Petrolini B, Borgonovi GE, Merli S. Isolation of endophytic Streptomyces from surface-sterilized roots. Appl Environ Microbiol, 1992; 58:2691–3. CrossRef
Taechowisan T, Chaisaeng S, Phutdhawong WS. Antibacterial, antioxidant and anticancer activities of biphenyls from Streptomyces sp. BO-07; an endophyte in Boesenbergia rotunda (L.) Mansf A. Food Agric Immunol, 2017; 28:1330–46. CrossRef
Taechowisan T, Peberdy JF, Lumyong S. Isolation of endophytic actinomycetes from selected plants and their antifungal activity. World J Microbiol Biotechnol, 2003; 19:381–5. CrossRef
Teresita SM, Hiroe K, Masashi H, Nobuji N. Constituents of Amomum tsao-ko and their radical scavenging and antioxidant activities. J Am Oil Chem Soc, 2000; 77(6):667–73. CrossRef
Wu HQ, Huang XL, Lin XS, Huang F, Ge FH. Analysis of the essential oils from Amomun kravak Pierre ex Gagnep by GC-MS. Zhong Yao Cai, 2006; 29(8): 788–92.
Wu L, Wu H, Chen L, Yu X, Borriss R, Gao X. Difficidin and bacilysin from Bacillus amyloliquefaciens FZB42 have antibacterial activity against Xanthomonas oryzae rice pathogens. Sci Rep, 2015; 5:12975. CrossRef
Yin H, Luo JG, Kong LY. Diarylheptanoids from the fruits of Amomum kravanh and their inhibitory activities of nitric oxide production. Phytochem Lett, 2013a; 6:403–6. CrossRef
Yin H, Luo JG, Kong LY. Tetracyclic diterpenoids with isomerized isospongian skeleton and labdane diterpenoids from the fruits of Amomum kravanh. J Nat Prod, 2013b; 76:237–42. CrossRef
Zeng Z, Fu L, Ye XN, Zhang T, Meng SJ, Meng CY. Comparison of volatile oil components from fructus amomi rotundus, fructus galangae, semen alpiniae katsumadai and semen myristicae. Chin J Appl Chem, 2012; 29:1316–23. CrossRef