INTRODUCTION
The association between sponges and sponge symbionts has potential in drug discovery. Not only are secondary metabolites produced by sponges, but also sponge symbionts may synthesize secondary metabolites. Therefore, the microbial symbionts associated with sponges can be isolated and cultured to increase the production of certain bioactive compounds derived from sponges (Lee et al., 2001). Marine sponge-derived fungi are a source of secondary metabolites that are currently being studied intensively. Although research on fungi derived from marine sponges is still less than research on terrestrial fungi, several important findings derived from fungi associated with marine sponges have added to the value of these fungi in the discovery of natural products (Butler et al., 2014), such as various kinds of secondary metabolites which have antimalarial, antiviral, antibacterial, and anticancer or cytotoxic activity. It is a potential that should be explored to increase the number of medicines derived from marine life without damaging the marine biota itself (Debbab et al., 2011; Hikmawan et al., 2020; Huang et al., 2011).
The metabolites produced by fungi coming from marine sponges are the result of chemical communication between fungus and sponge which is mutually beneficial; moreover, in some cases, they can produce completely new metabolites (Pejin and Karaman, 2017). The symbiosis between fungi and sponges, among others, provides a source of nutrition, a place of defense and protection, and stabilization of the sponge structure, treats sponge waste, and produces bioactive compounds. Therefore, it is likely that the bioactive compounds are also produced by the associated fungi of these sponges (Proksch et al., 2002; Thomas et al., 2010). Data obtained from the National Cancer Institutes of the United States show that sponges are a potential source of compounds in producing cytotoxic effects (Brinkmann et al., 2017). Thus, marine sponge-derived fungi also have the potential to produce bioactive compounds as cytotoxic.
To produce bioactive compounds, the associated fungi derived from marine sponges must be fermented outside the sponge’s body tissue using a fermentation media. The fermentation of microorganisms in this case is a fungus which will be influenced by physical and chemical factors. Physical factors affecting microorganisms comprise temperature, pH, and osmotic salinity, while chemical factors consist of sources of carbon, nitrogen, and nutrients in culture media (Pratiwi, 2008). The marine sponge-derived fungi depend on carbon, nitrogen, and salt (salinity) sources. Fungi associated with the marine environment based on several studies are strongly influenced by their growth in the presence of simple carbon sources, such as glucose and dextrose, and can even affect the production of secondary metabolites that affect their biological activity (Anuhya et al., 2017; Fuentes et al., 2015; Mahapatra et al., 2013; Miller et al., 1981; Ripa et al., 2009; Sguros and Simms, 1963). The increase in salt content (salinity) beyond seawater causes several species of associated fungi from the sea to decrease their growth rate, but the unavailability of salinity inhibits the growth of these associated fungi from the sea (Amon and Yei, 1982; Huang et al., 2011; Jones, 2000; Venkatachalam et al., 2019).
The objective of this review is to summarize the components extracted from the fermentation of marine sponge-derived fungi which have cytotoxic activity, identify the potential cytotoxic activity of these components based on the IC50 value, and find out what influences the fermentation conditions from marine sponge-derived fungi to produce cytotoxic bioactive compounds. The advantage of this review is to determine the relationship between the cytotoxic activity of fungi derived from marine sponges and their fermentation medium in producing cytotoxic compounds which have the potential to be developed as future cancer drug candidates. In the future, this review can be used as a reference to produce potential cytotoxic compounds from fungi from marine sponges using optimal fermentation medium conditions.
METHOD
A systematic search was conducted to find all publications related to the topic up to June 2020 on PubMed and Google Scholar. The keywords used to browse the articles were “fungi, sponge-derived, cytotoxic” or “fungi, sponge-associated, cancer”. The data included in this review were primary articles in English regarding cytotoxic studies of components produced from fungi derived from marine sponges and the conditions of fermentation, as shown in Table 1. Articles were excluded from primary articles if they were review articles, conference articles, and thesis, and no data were available for retrieval. All synthetic derivatives of natural metabolites that occur in sponges are not mentioned in this review. The variables assessed in this review include sponge species/genera of sponges, fungi-associated species/genera, fermentation medium, extracted specimens from fermentation products, components of the extracted product, type of cancer, type of cell line, and the cytotoxic effect of these components.
Cytotoxic activity of marine sponge-derived fungi
The number of articles that has been searched to June 2020 was 86 primary articles (Table 1). We identified that the 30 genera of sponge and 30 genera of fungi derived from the sponge investigated were related to their cytotoxic activity. The genera of sponges that are most frequently studied are Callyspongia, Halichondria, Phakellia, and Petrosia. The most frequently studied sponge-derived fungal genera are Aspergillus, Penicillium, Trichoderma, and Gymnascella. Figure 1 shows the number of studies that have been conducted for the cytotoxic activity of marine sponge-derived fungi, of which the number of publications is increasing from year to year. The highest number of publications published in 2019 was 14 articles, followed by 2018 and 2017 with 12 and 11 articles, respectively. The number of publications from 1997 to 2016 continued to increase, but the number of articles per year has not exceeded 2017–2019. It indicates that the focus of research in 1997–2016 was still on marine sponges explored for their bioactive components. Excessive exploration causes damage to the ecosystem of the sponge, which made the species decrease, and is not balanced with sponge growth. It creates a new trend, in which many scientists are interested in researching endophytic samples from sponges, including endophytic fungi from sponges, and it absolutely reduces the damage to sponge the habitat, which is increasingly rare in nature (Carroll et al., 2019; Thomas et al., 2010). Secondary metabolites from sea sponges have also been studied and have the potential in the medical world, including antiviral, antitumor, antimicrobial, antimalarial, and cytotoxicity (Guo et al., 2019; Hikmawan et al., 2020; Setyowati et al., 2009; Wang, 2006). Several studies have reported that the bioactive compounds obtained from sea sponges are most likely secondary metabolite compounds produced by the associated microbes in the bodies of marine sponges. It is caused by 40%–50% of the body tissue of marine sponges, which consists of microbes (Proksch et al., 2002; Thakur and Müller, 2004). The marine sponge association microbes can be fungi (Thomas et al., 2010).
Marine sponge-derived fungi produce bioactive compounds depending on the surrounding environment. The original habitat of these fungi is symbiotic in sponge tissues; thus, the production of compounds depends on the results of symbiosis with the host. When this fungus is outside its host, the active compound produced depends on the medium growth for the fungus. To be able to produce bioactive compounds similar to those produced in symbiosis with a sponge, the growth medium is made as closely as possible to the situation in its host. In addition to obtaining active compounds, it is also to reduce the occurrence of mutations that occur in fungi (Debbab et al., 2011; Huang et al., 2011; Kjer et al., 2010; Lee et al., 2001). Research on cytotoxic agents derived from marine sponges and their symbiotic microbes is still the concern of natural product researchers. More than 10% of cytotoxic activity comes from marine sponges that have been identified to date. The symbiosis of microorganisms from sponsors is proven to have an important role in bioactive compounds as cytotoxic agents. A review from 1955 to 2016 of marine sponges acting as cytotoxic agents reported that 107 new cytotoxic agents originated from marine sponges and were thought to have originated in symbiosis with the microbes present in these sponges (Zhang et al., 2017).
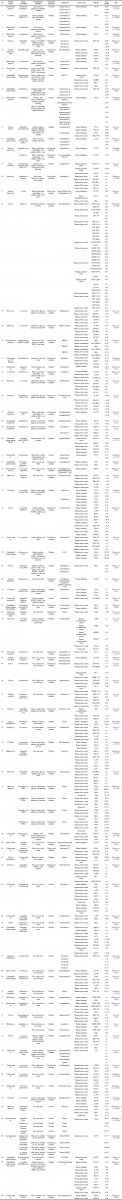 | Table 1. Summarized data of fermentation condition and cytotoxic activity from marine sponge-derived fungi. [Click here to view] |
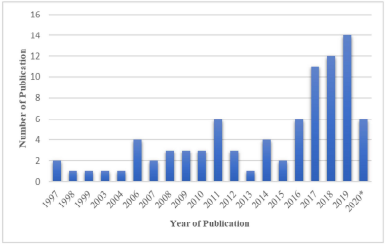 | Figure 1. Distribution of conducted studies about cytotoxic activity of marine sponge-derived fungi. *June 2020. [Click here to view] |
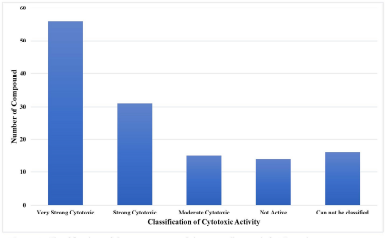 | Figure 2. Classification of the component activity according to their IC50 values. [Click here to view] |
Classification of cytotoxic activity of compound from marine sponge-derived fungi
In this review, we provide an overview of the bioactive metabolites extracted and isolated from marine sponge-derived fungi exhibiting in vitro cytotoxic activity in cell line cancer. By comparing the IC50 values, the units in nM, M, and ng/ml are converted into μg/ml unit by adjusting the molecular weight of the compound. All components were classified based on the IC50 value following the definition of Weerapreeyakul et al. (2012), which classifies the activity of cytotoxic components into “very strong cytotoxic”: IC50 is < 10 μg/ml; “strong cytotoxic”: IC50 is 10–100 μg/ml; and “moderate cytotoxic”: IC50 is 100–500 μg/ ml. What needs to be noted is that the test was administered with different cell line cancers, so there is a possibility that the inactive component in one cell line cancer could have a different IC50 value in another type of cell line cancer. It would be wise to reevaluate the activity of the inactive components obtained from marine sponge-derived fungi using other cancer cell lines (Badisa et al., 2009; Sutejo et al., 2016; Weerapreeyakul et al., 2012).
The bioactive components studied were 132 components extracted and isolated from marine sponge-derived fungi. These 132 components consist of 16 extracts, 5 fractions, and 111 isolates. As shown in Figure 2, among the observed bioactive components, there are 56 components with very strong cytotoxic activity, 31 components with strong cytotoxic activity, and 15 components with moderate cytotoxic activity against various cell line cancers. There are 16 components that cannot be classified because they have IC50 values > 500 μg/ml and have less accurate and clear IC50 values reported in the article. Furthermore, they cannot be included in that classification. There are 14 components reported to have no cytotoxic activity. The component is inactive only in some cell line cancers, and testing on other types of cell line cancers has not been conducted to see its cytotoxic activity (Badisa et al., 2009; Sutejo et al., 2016; Weerapreeyakul et al., 2012).
In this review, 27 types of cancer were found used in the study to determine the cytotoxic activity of marine sponge-derived fungi. The most common types of cancer are human leukemia, human colon cancer, human lung cancer, human cervix cancer, and human breast cancer. It is in line with the report by Bray et al. (2018) in which this type of cancer is reported to have a high incidence rate worldwide in humans, and even this type of cancer is included in the top 10 cancers causing death in humans. It has triggered many researchers to focus on these five types of cancer by exploring new compounds coming from the sea, especially marine sponge-derived fungi (Thomas et al., 2010). Potential compounds from marine sponge-derived fungi are expected to be used as new drugs in cancer treatment. To conduct a brief test of anticancer activity, researchers used in vitro cell line cancer to facilitate the screening of the anticancer activity of components obtained from marine sponge-derived fungi. In this review, we identified 317 types of cell line cancers used in determining the cytotoxic activity of components obtained from marine sponge-derived fungi. The most common types of cell line cancer are human cervix cancer (HeLa), human lung cancer (A-549), human leukemia (HL-60), mouse leukemia (P338), human liver cancer (HepG-2), human colon cancer (HCT-116), and human breast cancer (MCF-7). The use of this type of cell line cancer is based on the cancer incidence rates mentioned previously. Furthermore, there are factors which considered the use of this cell line cancer. These factors include easiness to handle and manipulate, high homogeneity, high degree of similarity with the initial tumor, large number and variety of cancer cell lines available, immediate accessibility to the scientific community, unlimited autoreplicative source, continuous cell lines, easy substitution of contaminated cultures for the respective frozen cell lines, and reproducibility of results in the correct conditions (Ferreira et al., 2013). Moreover, cell lines which are normal cells are also used. The use of normal cell lines aims to determine the cytotoxic strength of a sample that only damages cancer cells and does not damage normal cells of living things. The comparison of the IC50 value between normal cell line and cancer cell line produces a value called selectivity index (SI). Compounds or extracts having a SI > 3 have high selectivity in certain cancer cells (Badisa et al., 2009; Sutejo et al., 2016). The normal cell lines that are often used in cytotoxic-related research in this review are Vero (monkey epithelial kidney) and HL7702 (human normal liver).
The components isolated from marine sponge-derived fungi classified as very strong cytotoxic are shown in Table 2. Xanthone derivatives are metabolites generally distributed in higher plants and several types of fungi. This metabolite has several biological activities, such as antimicrobial, antiviral, antitubercular, and anticancer. Anomalin A (1) is one of the xanthones derived from the fungus Arthrinium sp. which is associated with the sponge Geodia cydonium (see Fig. 3) (Abdel-Lateff et al., 2003; Ebada et al., 2011; Morel et al., 2000; Peres et al., 2000).
 | Table 2. List of compounds with very strong cytotoxic activity based on the IC50 value. [Click here to view] |
Bioactive polyketide derivatives include bromophilone A (2), epoxyphomalin A (6), heterocornol A (9), heterocornol O (10), and oxalicumone A (14) (Fig. 3). Bromophilone A (2) is a polyketide azaphilone group with a bicyclic core and conjugated chromophore which has a bromide atom as a substituent. This metabolite is the combined result of the fungal fermentation media of Penicillium canescens with NaBr. Epoxyphomalin A (6) derived from the fungus Phoma sp. associated with the sponge Ectyplasia perox is a very active component in many cancer cell lines and this component has the potential to be developed for future cancer therapy. Heterocornol A (9) and heterocornol O (10) are polyketide derivatives derived from the fungi of the genera Pestalotiopsis associated with the sponge Phakellia fusca. Both these components have an IC50 ranging from 2 to 10 µg/ml in some cancer cells. Oxalicumone A (14) is a chromone-type bioactive polyketide derivative to be precise, dihydrothiophene-condensed chromone. This bioactive component comes from Aspergillus sp. LS34 associated with the sponge Haliclona sp. possessing very strong cytotoxic activity in the cancer cell lines CCRF-CEM and K562 (Frank et al., 2019; Gao et al., 2013; Lei et al., 2017a, 2019; Li et al., 2019; Mohamed et al., 2009; Sun et al., 2013; Wang et al., 2018).
Cytochalasins are a group of metabolites that are often found in fungi in several genera, such as Phomopsis, Chalara, Hyposylon, Xylaria, Daldinia, Pseudeurotium, and Phoma exigua. Cytochalasin K (3) is a metabolite of the marine sponge-derived fungi Arthrinium arundinis ZSDS1-F3 (Fig. 3). This class of metabolites is unique in its structure with a macrocyclic ring with antitumor, antibacterial, and HIV-1 protease inhibition activity. The most unusual activity of this metabolite is the ability to make the cell secrete its nucleus resulting in the formation of a cell without a nucleus (Liu et al., 2006; Wang et al., 2015). Diorcinols L (4) is a phenol derivative metabolite (Fig. 3). This metabolite comes from the fungus Didymellaceae sp. SCSIO F46 in association with Callyspongia sp. sponge. These phenol derivatives have strong cytotoxic activity in a number of cancer cell lines, including A-549, DU145, H1975, HeLa, HL60, Huh-7, and MCF-7 (Tian et al., 2018a). Acremonium sp. associated with the Teichaxinella sp. sponge produces several types of bioactive metabolite groups from the polyketides, hydroquinones, ketideterpenes, alkaloids, and terpene glycosides. Efrapeptin G (5) is a new bioactive metabolite from the peptaibiotic class derived from the fungus (Fig. 3). This bioactive metabolite has a very strong cytotoxic activity in several cancer cell lines, such as HCT-116, HeLa, HT-29, and SK-BR3 (Boot et al., 2006).
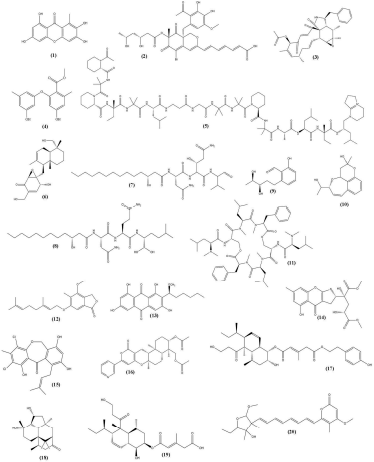 | Figure 3. Structure of a very strong cytotoxic component (Almeida et al., 2011; Boot et al., 2006; Cao et al., 2017; Cruz et al., 2006; Ebada et al., 2011; Frank et al., 2019; Lee et al., 2010a, 2010b, 2011; Lei et al., 2017a, 2017b, 2019; Li et al., 2019; Mohamed et al., 2009; Suzue et al., 2016; Tian et al., 2018a; Wang et al., 2015; Wu et al., 2015; Yamada et al., 2014, 2017). [Click here to view] |
Fellutamide C (7) and fellutamide F (8), components belonging to the lipopeptide group, have an IC50 of 0.1–3 µg/ml (Fig. 3). Both are derived from the fermentation of the fungus Aspergillus versicolor which is associated with the sponge Petrosia sp. growing on the coast of Jeju Island, Korea (Lee et al., 2010a; Lee et al., 2011). The new bioactive component IB-01212 (11) occurring from the fungus Clonostachys sp. ESNA-A009 has a very strong cytotoxic activity with an IC50 0.01 µg/ml (Fig. 3). This compound belongs to the cyclodepsipeptide group. Currently, IB-01212 (11) is being developed for biosynthetic so that it can be mass-produced without isolating it from fungi (Cruz et al., 2006). The bioactive phthalides group has activities such as a modulation of the central nervous system, protection against brain ischemia, modulation of platelet aggregation and heart function, inhibition of smooth muscle cell proliferation, antiangina activity, and smooth muscle relaxation, as well as antibacterial, antifungal, antiviral, and phytotoxic activity. Phthalides are secondary metabolites produced naturally by several types of fungi that are associated with the marine ecosystems, such as Ascochyta, Aspergillus, Alternaria, Penicillium, Hericium, or Talaromyces. Stachylidium sp. are associated with the sponge Callyspongia sp. cf. C. flammea producing marilone C (12) components included in the phthalides group (Fig. 3) (Almeida et al., 2011).
Aspergillus versicolor associated with the sponge Petrosia sp. produces the bioactive component methyl averantin (13) (Fig. 3). This secondary metabolite is included in the anthraquinone group. Methyl averantin (13) has very strong cytotoxic activity with an IC50 range 0.4–1.1 µg/ml in cancer cell lines like A-549, HCT-15, SK-MEL-2, SK-OV-3, and XF-498 (Lee et al., 2010b). As one of the most widespread genera of endophytic fungi, Pestalotiopsis produces various bioactive secondary metabolites. Pestalachloride B (15) is a metabolite of the fungal species Pestalotiopsis heterocornis associated with P. fusca which has cytotoxic activity with IC50 ranging from 2 to 10 µg/ml in cancer cell lines BCG-823, NCI-H460, and SMMC-7721 (Fig. 3) (Lei et al., 2017b; Li et al., 2008). Pyripyropene O (16) and trichodermanin C (18) are components belonging to the terpenes group (Fig. 3). Pyripyropene O (16) is pyripyropenes derived from sesquiterpenes conjugated with a-pyrone and pyridine moieties. Pyripyropenes are representative metabolites of several genera of fungi, such as Aspergillus and Penicillium. The bioactive metabolite pyripyropene O (16) is derived from Fusarium lateritium 2016F18-1 which is associated with the sponge Phyllospongia foliascens. Trichodermanin C (18) is classified as terpene with a rare fused 6-5-6-6 ring system. This bioactive metabolite comes from the fungus Trichoderma harzianum OUPS-111D-4 associated with the Halichondria okadai sponge (Cao et al., 2017; Yamada et al., 2017).
The secondary metabolites with an alkylated decalin skeleton have various bioactivities, such as antibacterial, antifungal, and phytotoxicity. There are many decalin derivatives including tandyukisin E (17) and trichoharzin (19) (Fig. 3). These two components are bioactive metabolites of the fungus T. harzianum OUPS-111D-4 associated with the H. okadai sponge. Tandyukisin E (17) has a unique chemical structure with a different side chain from the tandyukisin obtained so far and has cytotoxic activity in cancer cell lines HL-60, L1210, and P338 with IC50 values of 2.22, 3.59, and 2.17 µg/ml, respectively. Trichoharzin (19) is a polyketide constructed with an alkylated decalin skeleton and esterified with 3-methylglutaconic acid, a rare acyl moiety. This bioactive metabolite has cytotoxic activity in cell line HL-60 with IC50 = 6.66 µg/ml (Kobayashi et al., 1993; Suzue et al., 2016; Yamada et al., 2014). Emericella variecolor associated with the sponge Cinachyrella sp. produces several metabolites of the lactones group. Varioxiranol K (20) is one of the bioactive metabolites of this fungus (Fig. 3). These bioactive metabolites have very strong cytotoxic activity in cancer cell lines like A2780, BGC-823, HCT-116, HepG-2, and NCI-H1650 with an IC50 range of 1–4 µg/ml (Wu et al., 2015).
Influences of the fermentation conditions from marine sponge-derived fungi to produce cytotoxic metabolite
Metabolites produced by microbes are divided into two, primary metabolites and secondary metabolites. The production of primary metabolites is considered important, for instance, ethanol, citric acid, polysaccharides, acetone, butanol, and vitamins. Secondary metabolites produced by microbes include antibiotics, growth promoters, enzyme inhibitors, and others (Stanbury et al., 1995). Marine sponge-derived fungi produce a large number of new bioactive secondary metabolites, some of which exhibit new molecular structures that have never been previously found in nature. To be able to produce bioactive metabolites, the fungi associated with the sponge must first be isolated from the host and then fermented with a liquid medium of which composition is as close as possible to the state when it is in the host (Kjer et al., 2010).
In this review, we identified 11 types of carbon sources used in the fermentation media for marine sponge-derived fungi, including rice (38 media), glucose (33 media), malt extract (30 media), dextrose (9 media), and potato (8 media). Fungi require a greater amount of carbon than other essential elements because half of the dry weight of the fungal cell is estimated to consist of carbon which is important in the formation of the fungal cell wall (Moore-Landecker, 1996). The source of complex carbon in the medium is converted by the fungus into a simpler form that can be metabolized. Currently, rice and malt extract is widely used by researchers as a source of carbon in the medium. These two complex carbon sources, after being sterilized by heating, split into simpler carbon which could be used by fungi in their metabolism, with the result that these two carbon sources are widely used in the protocol for fermentation of marine sponge-derived fungi to produce new bioactive compounds, especially those useful for cancer (Kjer et al., 2010; Muthukumar et al., 2013). Some of the fungal isolates associated with the marine environment include Culcitalna achraspora Meyers and Moore, Humicola alopallonella Meyers and Moore, Orbomyces spectabilis Linder, Halosphaeria mediosetigera Cribb and Cribb, Penicillium decumbens, Penicillium chrysogenum, Acremonium strictum, Fusarium fujikuroi, and Fusarium sporotrichioides, which have a dry weight of mycelia developing with increasing levels of carbon sources in the fermentation media (Fuentes et al., 2015; Sguros and Simms, 1963). Trichoderma lignorum has increased conidia and hyphae growth when the carbon source is increased, but its growth decreases when the concentration of the carbon source exceeds 10 times of the frequent use (Seto and Tazaki, 1975). The effect of various carbon sources on the growth of Trichoderma viride species shows that the maximum production of secondary metabolites resulting from the highest to lowest production is influenced by the carbon sources of sucrose, glucose, cellulose, maltose, and carboxymethyl cellulose with an optimum level of 1%–1.5% (Gautam et al., 2010).
There are five types of nitrogen sources that we can identify in this review including peptone (30 media), yeast extract (16 media), glutamate (6 media), NaNO3 (2 media), and tryptophan (2 media). The nitrogen source in the marine sponge-derived fungi fermentation media does not appear to be present in the medium given its use which is not as much as the carbon source. Nitrogen sources are one of the important elements in the growth of endophytic fungi; however, nitrogen sources are not very influential in the growth of fungi and in the formation of secondary metabolites, except for metabolites containing nitrogen in their molecules. The use of peptone as a nitrogen source in the medium gives a high increase in mycelia dry weight compared to the use of inorganic nitrogen sources such as NaNO3 and NH4Cl (Hussain et al., 2003; Khattabi et al., 2004; Muthukumar et al., 2013).
Halophilic microorganisms can grow at high levels of salinity, for instance, in the sea with 3% NaCl. The salinity of a microorganism growth environment causes differences in osmotic pressure. Increasing levels of salinity exceeding the salinity of seawater resulted in several species of associated fungi from the sea decreasing their growth rates, but the unavailability of salinity inhibits the growth of these associated fungi from the sea. Several genera of associated fungi including Penicillium (32 strains), Aspergillus (10 strains), Mycelia sterilia (3 strains), Fusarium (1 strain), and Paecilomyces (1 strain) were isolated from several samples, such as seaweed, underwater sediments, and mangrove roots which have optimal growth and have the widest colony diameter found in fungi grown on medium with 3%–6% NaCl. The antimicrobial activity of these associated fungi in C. albicans showed the highest activity when treated with 6%–9% NaCl (Huang et al., 2011). The use of seawater or sea salt in the marine sponge-derived fungi fermentation media is very important because when living in its host, the surrounding environment of the fungus is a sea with salinity levels adjusting to the surrounding sea conditions. In this review, we identified 70 fermentation media using conditions such as in the sea, using natural seawater and sea salt, artificial seawater, and sea salt, while 26 media do not use conditions such as the origin of the fungus. The identification results show that the medium using seawater components (natural or artificial) produces bioactive components with very strong cytotoxic activity. The addition of components such as KH2PO4, MgSO4, MnCl2, and KCl in a medium which do not use seawater components also makes the fungi produce active metabolites as cytotoxic. Several genera of Trichoderma associated with the marine environment have optimal growth and dry weight mycelial at salinity levels of 1%–2%, but salinity levels that exceed 3% reduce the growth of fungal colonies (Bheemaraya et al., 2013; Mishra et al., 2016; Sánchez-Montesinos et al., 2019).
In this review, we classify the extracted specimens to obtain bioactive components from marine sponge-derived fungi into medium part, part mycelia, and both. The extraction process using both parts of mycelia and medium is the decision that most researchers do to extract bioactive components from fermentation. It is possible because the bioactive components are not yet known whether they are in the fungal cell or excreted out of the cell; therefore, the use of the extraction process for these two parts results in an optimal extraction. Furthermore, the extraction results using these two parts on average produce bioactive components containing a very strong cytotoxic activity (Kjer et al., 2010).
CONCLUSION
The data presented in the review show the potential of marine sponge-derived fungi as producing metabolites with cytotoxic activity and can reduce exploitation of rare sponges to produce bioactive components in cancer therapy. The components have been summarized and the most promising components are polyketide derivatives, lipopeptides, cyclodepsipeptides, decalin derivatives, xanthone derivatives, phenol derivatives, cytochalasins, peptaibiotics, phthalides, anthraquinone, terpenes, decalin derivatives, and lactones. In producing bioactive metabolites for cytotoxicity, the fermentation media is essential. Carbon sources, nitrogen, salinity, and extracted specimens are factors in the production of bioactive metabolites for cytotoxic fungi from marine sponges. A comprehensive approach is needed to evaluate the specific mechanism of action of the bioactive component as an anticancer. For further large-scale development in evaluating the production of bioactive metabolites from marine sponge-derived fungi, it may be necessary to develop components of the fermentation media which are more specific to certain fungi.
ACKNOWLEDGMENTS
The author would like to acknowledge the funding support from UGM no. 2488/UN1.P.III/DIT-LIT/PT/2020.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
CONFLICTS OF INTEREST
The authors report no conflicts of interest in this work.
ETHICAL APPROVAL
This study does not involve the use of animals or human subjects.
REFERENCES
Abdel-Lateff A, Klemke C, König GM, Wright AD. Two new xanthone derivatives from the algicolous marine fungus Wardomyces anomalus. J Nat Prod, 2003; 66(5):706–8. CrossRef
Almeida C, Kehraus S, Prudêncio M, König GM. Marilones A-C, phthalides from the sponge-derived fungus Stachylidium sp. Beilstein J Org Chem, 2011; 7:1636–42. CrossRef
Amagata T, Doi M, Tohgo M, Minoura K, Numata A. Dankasterone, a new class of cytotoxic steroid produced by a Gymnascella species from a marine sponge. Chem Commun, 1999; 1321–2. CrossRef
Amagata T, Minoura K, Numata A. Gymnastatins FH, Cytostatic metabolites from the sponge-derived fungus Gymnascella dankaliensis. J Nat Prod, 2006; 69(10):1384–8. CrossRef
Amagata T, Minoura K, Numata A. Gymnasterones, novel cytotoxic metabolites produced by a fungal strain from a sponge. Tetrahedron Lett, 1998a; 39:3773–4. CrossRef
Amagata T, Tanaka M, Yamada T, Chen YP, Minoura K, Numata A. Additional cytotoxic substances isolated from the sponge-derived Gymnascella dankaliensis. Tetrahedron Lett, 2013; 54(45):5960–2. CrossRef
Amagata T, Tanaka M, Yamada T, Doi M, Minoura K, Ohishi H, Yamori T, Numata A. Variation in cytostatic constituents of a sponge-derived Gymnascella dankaliensis by manipulating the carbon source. J Nat Prod, 2007; 70(11):1731–40. CrossRef
Amagata T, Usami Y, Minoura K, Ito T, Numata A. Cytotoxic substances produced by a fungal strain from a sponge: physico-chemical properties and structures. J Antibiot (Tokyo), 1998b; 51(1):33–40. CrossRef
Amon JP, Yei S. The effect of salinity on the growth of two marine fungi in mixed culture. Mycologia, 1982; 74(1):117–22. CrossRef
Anuhya G, Jyostna V, Aswani KY, Bodaiah B, Sudhakar P. Influence of physico-chemical parameters on secondary metabolite production by marine fungi. Int J Curr Pharm Res, 2017; 9(5):112–8. CrossRef
Artasasta MA, Taher M, Djamaan A, Handayani D. Cytotoxic and antibacterial activities of marine sponge-derived fungus Aspergillus nomius NC06. Rasayan J Chem, 2019; 12(3):1463–9. CrossRef
Artasasta MA, Yanwirasti, Djamaan A, Handayani D. Cytotoxic activity screening of ethyl acetate fungal extracts derived from the marine sponge Neopetrosia chaliniformis AR-01. J Appl Pharm Sci, 2017; 7(12):174–8.
Badisa RB, Darling-Reed SF, Joseph P, Cooperwood JS, Latinwo LM, Goodman CB. Selective cytotoxic activities of two novel synthetic drugs on human breast carcinoma MCF-7 cells. Anticancer Res, 2009; 29(8):2993–6.
Bheemaraya PMB, Ramesh YST, Amaresh YS, Naik MK. Salinity stress tolerance in native Trichoderma isolates. Environ Ecol, 2013; 31(2A):727–9.
Boot CM, Tenney K, Valeriote FA, Crews P. Highly N-methylated linear peptides produced by an atypical sponge-derived Acremonium sp. J Nat Prod, 2006; 69(1):83–92. CrossRef
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin, 2018; 68(6):394–424. CrossRef
Brinkmann CM, Marker A, Kurtböke DI. An overview on marine sponge-symbiotic bacteria as unexhausted sources for natural product discovery. Diversity, 2017; 9(40):1–31. CrossRef
Bugni TS, Bernan VS, Greenstein M, Janso JE, Maiese WM, Mayne CL, Ireland CM. Brocaenols A-C: novel polyketides from a marine-derived Penicillium brocae. J Org Chem, 2003; 68(5):2014–7. CrossRef
Butler MS, Robertson AAB, Cooper MA. Natural product and natural product derived drugs in clinical trials. Nat Prod Rep, 2014; 31(11):1612–61. CrossRef
Buttachon S, Ramos AA, Inácio Â, Dethoup T, Gales L, Lee M, Costa PM, Silva AMS, Sekeroglu N, Rocha E, Pinto MMM, Pereira JA, Kijjoa A. Bis-indolyl benzenoids, hydroxypyrrolidine derivatives and other constituents from cultures of the marine sponge-associated fungus Aspergillus candidus KUFA0062. Mar Drugs, 2018; 16(4):1–22. CrossRef
Cao QX, Wei JH, Deng R, Feng GK, Zhu XF, Lan WJ, Li HJ. Two new pyripyropenes from the marine fungus Fusarium lateritium 2016F18-1. Chem Biodivers, 2017; 14(3):1–6. CrossRef
Carroll AR, Copp BR, Davis RA, Keyzers RA, Prinsep MR. Marine natural products. Nat Prod Rep, 2019; 36(1):122–73. CrossRef
Chen Y, Mao WJ, Yan MX, Liu X, Wang SY, Xia Z, Xiao B, Cao SJ, Yang BQ, Li J. Purification, chemical characterization, and bioactivity of an extracellular polysaccharide produced by the marine sponge endogenous fungus Alternaria sp. SP-32. Mar Biotechnol, 2016; 18(3):301–13. CrossRef
Cohen E, Koch L, Thu KM, Rahamim Y, Aluma Y, Ilan M, Yarden O, Carmeli S. Novel terpenoids of the fungus Aspergillus insuetus isolated from the mediterranean sponge Psammocinia sp. collected along the coast of Israel. Bioorg Med Chem, 2011; 19(22):6587–93. CrossRef
Cruz LJ, Martínez Insua M, Pérez Baz J, Trujillo M, Rodriguez-Mias RA, Oliveira E, Giralt E, Albericio F, Cañedo LM. IB-01212, a new cytotoxic cyclodepsipeptide isolated from the marine fungus Clonostachys sp. ESNA-A009. J Org Chem, 2006; 71(9):3335–8. CrossRef
Cueto M, MacMillan JB, Jensen PR, Fenical W. Tropolactones A-D, four meroterpenoids from a darine-derived fungus of the genus Aspergillus. Phytochemistry, 2006; 67(16):1826–31. CrossRef
Debbab A, Aly AH, Proksch P. Bioactive secondary metabolites from endophytes and associated marine derived fungi. Fungal Divers, 2011; 49(1):1–12. CrossRef
Ding LJ, Yuan W, Liao XJ, Han BN, Wang SP, Li ZY, Xu SH, Zhang W, Lin HW. Oryzamides A−E, Cyclodepsipeptides from the sponge-derived fungus Nigrospora oryzae PF18. J Nat Prod, 2016; 79(8):2045–52. CrossRef
Ebada SS, Schulz B, Wray V, Totzke F, Kubbutat MHG, Müller WEG, Hamacher A, Kassack MU, Lin W, Proksch P. Arthrinins A-D: novel diterpenoids and further constituents from the sponge derived fungus Arthrinium sp. Bioorg Med Chem, 2011; 19(15):4644–51. CrossRef
Elbandy M, Shinde PB, Hong J, Bae KS, Kim MA, Lee SM, Jung JH. α-Pyrones and yellow pigments from the sponge-derived fungus Paecilomyces lilacinus. Bull Korean Chem Soc, 2009; 30(1):188–92. CrossRef
Elissawy AM, Ebada SS, Ashour ML, Özkaya FC, Ebrahim W, Singab ANB, Proksch P. Spiroarthrinols A and B, two novel meroterpenoids isolated from the sponge-derived fungus Arthrinium sp. Phytochem Lett, 2017; 20:246–51. CrossRef
Ferreira D, Adega F, Chaves R. The importance of cancer cell lines as in vitro models in cancer methylome analysis and anticancer drugs testing. In: López-Camarillo C, Aréchaga-Ocampo E. (ed.). Oncogenomics and cancer proteomics - novel approaches in biomarkers discovery and therapeutic targets in cancer. InTech Prepress, Rijeka, Croatia, 2013. CrossRef
Frank M, Hartmann R, Plenker M, Mándi A, Kurtán T, Özkaya FC, Müller, Werner EG, Kassack MU, Hamacher A, Lin W, Liu Z, Proksch P. Brominated Azaphilones from the sponge-associated fungus Penicillium canescens strain 4.14.6a. J Nat Prod, 2019; 82(8):1–8. CrossRef
Fuentes ME, Quiñones RA, Gutiérrez MH, Pantoja S. Effects of temperature and glucose concentration on the growth and respiration of fungal species isolated from a highly productive coastal upwelling ecosystem. Fungal Ecol, 2015; 13(2015):135–49. CrossRef
Gao J, Yang S, Qin J. Azaphilonoids: chemistry and biology. Chem Rev, 2013; 113(7):4755–811. CrossRef
Gautam SP, Bundela PS, Pandey AK, Jamaluddin, Awasthi MK, Sarsaiya S. Optimization of the medium for the production of cellulase by the Trichoderma viride using submerged fermentation. Int J Environ Sci, 2010; 1(4):656–65.
Gu Y, Ding P, Liang Z, Song Y, Liu Y, Chen G, Li JL. Activated production of silent metabolites from marine-derived fungus Penicillium citrinum. Fitoterapia 2018; 127:207–11. CrossRef
Guo C, Wang P, Lin X, Salendra L, Kong F, Liao S, Yang B, Zhou X, Wang J, Liu Y. Phloroglucinol heterodimers and bis-indolyl alkaloids from the sponge-derived fungus: Aspergillus sp. SCSIO 41018. Org Chem Front, 2019; 6(17):3053–9. CrossRef
Handayani D, Artasasta MA, Safirna N, Ayuni DF, Tallei TE, Hertiani T. Fungal isolates from marine sponge Chelonaplysilla sp.: diversity, antimicrobial and cytotoxic activities. Biodiversitas, 2020a; 21(5):1954–60. CrossRef
Handayani D, Putri RA, Ismed F, Hertiani T, Ariantari NP, Proksch P. Bioactive metabolite from marine sponge-derived fungus Cochliobolus geniculatus WR12. Rasayan J Chem, 2020b; 13(1):417–22. CrossRef
Handayani D, Rasyid W, Rustini, Zainudin EN, Hertiani T. Cytotoxic activity screening of fungal extracts derived from the West Sumatran marine sponge Haliclona fascigera to several human cell lines: hela, WiDr, T47D and vero. J Appl Pharm Sci, 2018; 8(1):55–8.
Heydari H, Koc A, Simsek D, Gozcelioglu B, Altanlar N, Konuklugil B. Isolation, identification and bioactivity screening of Turkish marine-derived fungi. Farmacia, 2019; 67(5):780–8. CrossRef
Hikmawan BD, Wahyuono S, Setyowati EP. Review: marine sponge compounds with antiplasmodial properties: focus on in vitro studies against Plasmodium falciparum. J Appl Pharm Sci, 2020; 10(5):142–57. CrossRef
Hiort J, Maksimenka K, Reichert M, Perović-Ottstadt S, Lin WH, Wray V, Steube K, Schaumann K, Weber H, Proksch P, Ebel R, Müller WEG, Bringmann G.. New natural products from the sponge-derived fungus Aspergillus niger. J Nat Prod, 2004; 67(9):1532–43. CrossRef
Huang J, Lu C, Qian X, Huang Y, Zheng Z, Shen Y. Effect of salinity on the growth, biological activity and secondary metabolites of some marine fungi. Acta Oceanol Sin, 2011; 30(3):118–23. CrossRef
Huang L, Ding L, Li X, Wang N, Cui W, Wang X, Naman CB, Lazaro JEH, Yan X, He S. New Dihydroisocoumarin root growth inhibitors from the sponge-derived fungus Aspergillus sp. NBUF87. Front Microbiol, 2019a; 10:1–10. CrossRef
Huang L, Ding L, Li X, Wang N, Yan Y, Yang M, Cui W, Benjamin NC, Cheng K, Zhang W, Zhang B, Jin H, He S. A new lateral root growth inhibitor from the sponge-derived fungus Aspergillus sp. LS45. Bioorg Med Chem Lett, 2019b; 29(13):1593–6. CrossRef
Hussain A, Iqbal SM, Ayub N, Haqqani AMH. Physiological study of Sclerotium rolfsii sacc. Pak J Plant Pathol, 2003; 2(2):102–6. CrossRef
Ibrahim AH, Attia EZ, Hajjar D, Anany MA, Desoukey SY, Fouad MA, Kamel MS, Wajant H, Gulder TAM, Abdelmohsen UR. New cytotoxic cyclic peptide from the marine sponge-associated Nocardiopsis sp. Ur67. Mar Drugs, 2018; 16(9):1–13. CrossRef
Jones EBG. Marine fungi: some factors influencing biodiversity. Fungal Divers, 2000; 4:53–73.
Khattabi N, Ezzahiri B, Louali L, Oihabi A. Effect of nitrogen fertilizers and Trichoderma harzianum on Sclerotium rolfsii. Agronomie, 2004; 24:281–8. CrossRef
Kito K, Ookura R, Yoshida S, Namikoshi M, Ooi T, Kusumi T. New cytotoxic 14-membered macrolides from marine-derived fungus Aspergillus ostianus. Org Lett, 2008; 10(2):225–8. CrossRef
Kjer J, Debbab A, Aly AH, Proksch P. Methods for isolation of marine-derived endophytic fungi and their bioactive secondary products. Nat Protoc, 2010; 5(3):479–90. CrossRef
Kobayashi M, Uehara H, Matsunami K, Aoki S, Kitagawa I. Trichoharzin, a new polyketide produced by the imperfect fungus Trichoderma harzianum separated from the marine sponge Micale cecilia. Tetrahedron Lett, 1993; 34(49):7925–8. CrossRef
Küppers L, Ebrahim W, El-Neketi M, Özkaya FC, Mándi A, Kurtán T, Orfali RS, Müller WEG, Hartmann R, Lin W. Lactones from the sponge-derived fungus Talaromyces rugulosus. Mar Drugs, 2017; 15(359):1–16. CrossRef
Lee YK, Lee J, Lee HK. Minireview: microbial symbiosis in marine sponges. J Microbiol, 2001; 39(4):254–64.
Lee YM, Dang HT, Hong J, Lee CO, Bae KS, Kim DK, Jung JH. A cytotoxic lipopeptide from the sponge-derived fungus Aspergillus versicolor. Bull Korean Chem Soc, 2010a; 31(1):205–8. CrossRef
Lee YM, Dang HT, Li J, Zhang P, Hong J, Lee CO, Jung JH. A cytotoxic fellutamide analogue from the sponge-derived fungus Aspergillus versicolor. Bull Korean Chem Soc, 2011; 32(10):3817–20. CrossRef
Lee YM, Li H, Hong J, Cho HY, Bae KS, Kim MA, Kim DK, Jung JH. Bioactive metabolites from the sponge-derived fungus Aspergillus versicolor. Arch Pharm Res, 2010b; 33(2):231–5. CrossRef
Lei H, Lei J, Zhou X, Hu M, Niu H, Song C, Chen S, Liu Y, Zhang D. Cytotoxic polyketides from the marine sponge-derived fungus Pestalotiopsis heterocornis XWS03F09. Molecules, 2019; 24(2655):1–8. CrossRef
Lei H, Lin X, Han L, Ma J, Dong K, Wang X, Zhong J, Mu Y, Liu Y, Huang X. Polyketide derivatives from a marine-sponge-associated fungus Pestalotiopsis heterocornis. Phytochemistry, 2017a; 142:51–9. CrossRef
Lei H, Lin X, Han L, Ma J, Ma Q, Zhong J, Liu Y, Sun T, Wang J, Huang X. New metabolites and bioactive chlorinated benzophenone derivatives produced by a marine-derived fungus Pestalotiopsis heterocornis. Mar Drugs, 2017b; 15(69):1–10. CrossRef
Li D, Xu Y, Shao CL, Yang RY, Zheng CJ, Chen YY, Fu XM, Qian PY, She ZG, De Voogd NJ, Wang CY. Antibacterial bisabolane-type sesquiterpenoids from the sponge-derived fungus Aspergillus sp. Mar Drugs, 2012; 10(1):234–41. CrossRef
Li E, Jiang L, Guo L, Zhang H, Che Y. Pestalachlorides A-C, antifungal metabolites from the plant endophytic fungus Pestalotiopsis adusta. Bioorg Med Chem, 2008; 16(17):7894–9. CrossRef
Li JL, Zhang P, Lee YM, Hong J, Yoo ES, Bae KS, Jung JH. Oxygenated hexylitaconates from a marine sponge-derived fungus Penicillium sp. Chem Pharm Bull, 2011; 59(1):120–3. CrossRef
Li W, Ding L, Wang N, Xu J, Zhang W, Zhang B, He S, Wu B, Jin H. Isolation and characterization of two new metabolites from the sponge-derived fungus Aspergillus sp. LS34 by OSMAC approach. Mar Drugs, 2019; 17(283):1–9. CrossRef
Li Y, Liu D, Cheng Z, Proksch P, Lin W. Cytotoxic trichothecene-type sesquiterpenes from the sponge-derived fungus: Stachybotrys chartarum with tyrosine kinase inhibition. RSC Adv, 2017; 7(12):7259–67. CrossRef
Liu H, Edrada-Ebel R, Ebel R, Wang Y, Schulz B, Draeger S, Müller WEG, Wray V, Lin W, Proksch P. Drimane sesquiterpenoids from the fungus Aspergillus ustus isolated from the marine sponge Suberites domuncula. J Nat Prod, 2009; 72(9):1585–8. CrossRef
Liu HB, Edrada-Ebel R, Ebel R, Wang Y, Schulz B, Draeger S, Müller WEG, Wray V, Lin W, Proksch P. Ophiobolin sesterterpenoids and pyrrolidine alkaloids from the sponge-derived fungus Aspergillus ustus. Helv Chim Acta, 2011; 94(4):623–31. CrossRef
Liu N, Peng S, Yang J, Cong Z, Lin X, Liao S, Yang B, Zhou X, Zhou X, Liu Y, Wang J. Structurally diverse sesquiterpenoids and polyketides from a sponge-associated fungus Aspergillus sydowii SCSIO41301. Fitoterapia, 2019; 135:27–32. CrossRef
Liu R, Gu Q, Zhu W, Cui C, Fan G, Fang Y, Zhu T, Liu H. 10-Phenyl-[12]-cytochalasins Z7, Z8, and Z 9 from the marine-derived fungus Spicaria elegans. J Nat Prod, 2006; 69(6):871–5. CrossRef
Liu S, Wang H, Su M, Hwang GJ, Hong J, Jung JH. New metabolites from the sponge-derived Fungus Aspergillus sydowii J05B-7F-4. Nat Prod Res, 2017; 31(14):1–5. CrossRef
Luo XW, Chen C, Tao H, Lin X, Yang B, Zhou X, Liu Y. Structurally diverse diketopiperazine alkaloids from the marine-derived fungus: Aspergillus versicolor SCSIO 41016. Org Chem Front, 2019a; 6(6):1–5. CrossRef
Luo XW, Lin Y, Lu YJ, Zhou XF, Liu YH. Peptides and polyketides isolated from the marine sponge-derived fungus Aspergillus terreus SCSIO 41008. Chin J Nat Med, 2019b; 17(2):149–54. CrossRef
Mahapatra S, Banerjee D. Optimization of a bioactive exopolysaccharide production from endophytic Fusarium solani SD5. Carbohydr Polym, 2013; 97(2):627–34. CrossRef
Meng LH, Chen HQ, Form I, Konuklugil B, Proksch P, Wang BG. New chromone, isocoumarin, and indole alkaloid derivatives from three sponge-derived fungal strains. Nat Prod Commun, 2016; 11(9):1293–6. CrossRef
Miller JD, Whitney NJ. Fungi of the bay of fundy - III. Geofungi in the marine environment. Mar Biol, 1981; 65(1):61–8. CrossRef
Mishra N, Khan SS, Sundari SK. Native isolate of Trichoderma: a biocontrol agent with unique stress tolerance properties. World J Microbiol Biotechnol, 2016; 32(130):1–23. CrossRef
Mohamed LE, Gross H, Pontius A, Kehraus S, Krick A, Kelter G, Maier A, Fiebig HH, König GM. Epoxyphomalin A and B, prenylated polyketides with potent cytotoxicity from the marine-derived fungus Phoma sp. Org Lett, 2009; 11(21):5014–7. CrossRef
Moore-Landecker E. Fundamentals of the fungi. 4th edition, Upper Saddle River, NJ: Prentice Hall, 1996.
Morel C, Séraphin D, Oger JM, Litaudon M, Sévenet T, Richomme P, Bruneton J. New xanthones from Calophyllum caledonicum. J Nat Prod, 2000; 63(11):1471–4. CrossRef
Muthukumar A, Venkatesh A. Physiological studies of Sclerotium rolfsii Sacc. causing collar rot of peppermint. Afr J Biotechnol, 2013; 12(49):6837–42.
Numata A, Amagata T, Minoura K, Lto T. Gymnasterones, novel cytotoxic metabolites produced by a fungal strain from a sponge. Tetrahedron Lett, 1997; 38(32):5675–8. CrossRef
Özkaya FC, Ebrahim W, El-Neketi M, Tansel Tanrıkul T, Kalscheuer R, Müller WEG, Guo Z, Zou K, Liu Z, Proksch P. Induction of new metabolites from sponge-associated fungus Aspergillus carneus by OSMAC approach. Fitoterapia, 2018; 131(August):9–14. CrossRef
Pang X, Lin X, Wang P, Zhou X, Yang B, Wang J, Liu Y. Perylenequione derivatives with anticancer activities isolated from the marine sponge-derived fungus, Alternaria sp. SCSIO41014. Mar Drugs, 2018;16(280):1–13. CrossRef
Pejin B, Karaman M. Antitumor natural products of marine-derived fungi boris. Fungal Metab, 2017; 1–28. CrossRef
Peres V, Nagem TJ, de Oliveira FF. Review: tetraoxygenated naturally occurring xanthones. Phytochemistry, 2000; 55:683–710. CrossRef
Pratiwi SUT. Mikrobiologi farmasi. Erlangga, Jakarta, Indonesia, 2008.
Proksch P, Ebel R, Edrada R, Riebe F, Liu H, Diesel A, Bayer M, Li X, Han Lin W, Grebenyuk V, Müller WEG, Draeger S, Zuccaro A, Schulz B. Sponge-associated fungi and their bioactive compounds: the suberites case. Bot Mar, 2008; 51(3):209–18. CrossRef
Proksch P, Edrada RA, Ebel R. Drugs from the seas - current status and microbiological implications. Appl Microbiol Biotechnol, 2002; 59(2–3):125–34. CrossRef
Qin C, Lin X, Lu X, Wan J, Zhou X, Liao S, Tu Z, Xu S, Liu Y. Sesquiterpenoids and xanthones derivatives produced by sponge-derived fungus Stachybotry sp. HH1 ZSDS1F1-2. J Antibiot, 2014; 1–5. CrossRef
Ripa FA, Nikkon F, Zaman S, Khondkar P. Optimal conditions for antimicrobial metabolites production from a new Streptomyces sp. RUPA-08PR isolated from Bangladeshi soil. Mycobiology, 2009; 37(3):211. CrossRef
Salendra L, Lin X, Chen W, Pang X, Luo X, Long J, Liao S, Wang J, Zhou X, Liu Y, Yang B. Cytotoxicity of polyketides and steroids isolated from the sponge-associated fungus Penicillium citrinum SCSIO 41017. Nat Prod Res, 2019a; 1–9. CrossRef
Salendra L, Luo X, Lin X, Wang J, Yang B, Zhou X, Liu Y. Versispiroketal A, an unusual tetracyclic bridged spiroketal from the sponge-associated fungus Aspergillus versicolor SCSIO 41013. Org Biomol Chem, 2019b; 17(8):1–5. CrossRef
Sánchez-Montesinos B, Diánez F, Moreno-Gavira A, Gea FJ, Santos M. Plant growth promotion and biocontrol of Pythium ultimum by saline tolerant Trichoderma isolates under salinity stress. Int J Environ Res Public Health, 2019; 16(2053):1–11. CrossRef
Sandrawati N, Hati SP, Yunita F, Putra AE, Ismed F, Tallei TE, Hertiani T, Handayani D. Antimicrobial and cytotoxic activities of marine sponge-derived fungal extracts isolated from Dactylospongia sp. J Appl Pharm Sci, 2020; 10(4):28–33. CrossRef
Seto M, Tazaki T. Growth and respiratory activity of mold fungus (Trichoderma lignorum). Bot Mag, 1975; 88:255–66. CrossRef
Setyowati EP, Jenie UA, Sudarsono, Kardono LBS. Theonellapeptolide ld: structure identification of cytotoxic constituent from Kaliapsis sp. sponge (bowerbank) collected from West Bali Sea Indonesia. J Biol Sci, 2009; 9(1):29–36. CrossRef
Setyowati EP, Pratiwi SUT, Hertiani T, Samirana O. Bioactivity of fungi Trichoderma reesei, associated with sponges Stylissa flabelliformis collected from National Park West Bali, Indonesia. J Biol Sci, 2017; 17(8):362–8. CrossRef
Setyowati EP, Pratiwi SUT, Purwantiningsih, Purwantini I. Invitro cytotoxicity and apoptosis mechanism of ethyl acetate Extract from Trichoderma reesei strain TV221 associated with marine sponge: Stylissa flabelliformis. J Appl Pharm Sci, 2018; 8(9):151–7. CrossRef
Sguros P, Simms J. Role of marine fungi in the biochemistry of the oceans. II. Effect of glucose, inorganic nitrogen, and tris (hydroxymethyl) aminomethane on growth and Ph changes in synthetic media. Mycologia, 1963; 55(6):728–41. CrossRef
Shin HJ, Choi BK, Trinh PTH, Lee HS, Kang JS, Van TTT, Lee HS, Lee JS, Lee YJ, Lee J. Suppression of RANKL-induced osteoclastogenesis by the metabolites from the marine fungus Aspergillus flocculosus isolated from a sponge Stylissa sp. Mar Drugs, 2018; 16(14):1– 9. CrossRef
Stanbury PF, Whitaker A, Hall SJ. Principles of fermentation technology. 2nd édition, London, UK: Elsevier Ltd, 1995. CrossRef
Sun LL, Shao CL, Chen JF, Guo ZY, Fu XM, Chen M, Chen YY, Li R, De Voogd NJ, She ZG, Lin YC, Wang CY. New bisabolane sesquiterpenoids from a marine-derived fungus Aspergillus sp. isolated from the sponge Xestospongia testudinaria. Bioorg Med Chem Lett, 2012; 22(3):1326–9. CrossRef
Sun YL, Bao J, Liu KS, Zhang XY, He F, Wang YF, Nong XH, Qi SH. Cytotoxic dihydrothiophene-condensed chromones from the marine-derived fungus Penicillium oxalicum. Planta Med, 2013; 79(15):1474–9. CrossRef
Sureram S, Wiyakrutta S, Ngamrojanavanich N, Mahidol C, Ruchirawat S, Kittakoop P. Depsidones, aromatase inhibitors and radical scavenging agents from the marine-derived fungus Aspergillus unguis CRI282-03. Planta Med, 2012; 78(6):582–8. CrossRef
Sutejo IR, Putri H, Meiyanto E. The selectivity of ethanolic extract of buah makassar (Brucea javanica) on in vitro study of metastatic breast cancer. J Agromedicine Med Sci, 2016; 2(1):1–5. CrossRef
Suzue M, Kikuchi T, Tanaka R, Yamada T. Tandyukisins E and F, novel cytotoxic decalin derivatives isolated from a marine sponge-derived fungus. Tetrahedron Lett, 2016; 57(46):5070–3. CrossRef
Tang R, Kimishima A, Ishida R, Setiawan A, Arai M, Selective cytotoxicity of epidithiodiketopiperazine DC1149B, produced by marine-derived Trichoderma lixii on the cancer cells adapted to glucose starvation. J Nat Med, 2020; 74:153–8. CrossRef
Thakur NL, Müller WEG. Biotechnological potential of marine sponges. Curr Sci, 2004; 86(11):1506–12.
Thomas TRA, Kavlekar DP, LokaBharathi PA. Marine drugs from sponge-microbe association - a review. Mar Drugs, 2010; 8(4):1417– 68. CrossRef
Tian Y, Lin X, Zhou X, Liu Y. Phenol derivatives from the sponge-derived fungus Didymellaceae sp. SCSIO F46. Front Chem, 2018a; 6(536):1–8. CrossRef
Tian YQ, Lin SN, Zhou H, Lin ST, Wang SY, Liu YH. Protuboxepin C and protuboxepin D from the sponge-derived fungus Aspergillus sp. SCSIO XWS02F40. Nat Prod Res, 2018b; 32(21):1–6. CrossRef
Ueda JY, Takagi M, Shin-Ya K. New xanthoquinodin-like compounds, JBIR-97,-98 and-99, obtained from marine sponge-derived fungus Tritirachium sp. SpB081112MEf2. J Antibiot, 2010; 63(10):615–8. CrossRef
Venkatachalam M, Gérard L, Milhau C, Vinale F, Dufossé L, Fouillaud M. Salinity and temperature influence growth and pigment production in the marine-derived fungal strain Talaromyces albobiverticillius 30548. Microorganisms, 2019; 7(10):1–19. CrossRef
Wang D, Qu P, Zhou J, Wang Y, Wang L, Zhu W. p-Terphenyl alcohols from a marine sponge-derived fungus, Aspergillus candidus OUCMDZ-1051.pdf. Mar Life Sci Technol, 2020a; 2:262–7. CrossRef
Wang G. Diversity and biotechnological potential of the sponge-associated microbial consortia. J Ind Microbiol Biotechnol, 2006; 33(7):545–51. CrossRef
Wang J, Wang Z, Ju Z, Wan J, Liao S, Lin X, Zhang T, Zhou X, Chen H, Tu Z, Liu Y. Cytotoxic cytochalasins from marine-derived fungus Arthrinium arundinis. Planta Med, 2015; 81(2):160–6. CrossRef
Wang L, Jiao J, Liu D, Zhang X, Li J, Che Q, Zhu T, Zhang G, Li D. Cytotoxic meroterpenoids from the fungus Alternaria sp. JJY-32. Chem Biodivers, 2020b; 17(7):1–9. CrossRef
Wang W, Liao Y, Chen R, Hou Y, Ke W, Zhang B, Gao M, Shao Z, Chen J, Li F. Chlorinated azaphilone pigments with antimicrobial and cytotoxic activities isolated from the deep sea derived fungus Chaetomium sp. NA-S01-R1. Mar Drugs, 2018; 16(2):1–11. CrossRef
Wang X, Mou Y, Hu J, Wang N, Zhao L, Liu L, Wang S, Meng D. Cytotoxic polyphenols from a sponge-associated fungus Aspergillus versicolor Hmp-48. Chem Biodivers, 2014; 11(1):133–9. CrossRef
Weerapreeyakul N, Nonpunya A, Barusrux S, Thitimetharoch T, Sripanidkulchai B. Evaluation of the anticancer potential of six herbs against a hepatoma cell line. Chin Med, 2012; 7(15):1–7. CrossRef
Wu Q, Long HL, Liu D, Proksch P, Lin WH. Varioxiranols IL, New lactones from a sponge-associated Emericella variecolor fungus. J Asian Nat Prod Res, 2015; 17(12):1137–45. CrossRef
Wu Z, Li Y, Liu D, Ma M, Chen J, Lin W. New resorcinol derivatives from a sponge-derived fungus Hansfordia sinuosae. Chem Biodivers, 2017; 14(6):e1700059. CrossRef
Wu Z, Liu D, Proksch P, Guo P, Lin W. Punctaporonins H-M: caryophyllene-type sesquiterpenoids from the sponge-associated fungus Hansfordia sinuosae. Mar Drugs, 2014; 12(7):3904–16. CrossRef
Xin ZH, Fang Y, Du L, Zhu T, Duan L, Chen J, Gu QQ, Zhu WM. Aurantiomides A-C, quinazoline alkaloids from the sponge-derived fungus Penicillium aurantiogriseum SP0-19. J Nat Prod, 2007; 70(5):853–5. CrossRef
Yamada T, Fujii A, Kikuchi T. New diterpenes with a fused 6-5-6-6 ring system isolated from the marine sponge-derived fungus: Trichoderma harzianum. Mar Drugs, 2019; 17(480):1–10.
Yamada T, Mizutani Y, Umebayashi Y, Inno N, Kawashima M, Kikuchi T, Tanaka R. Tandyukisin, a novel ketoaldehyde decalin derivative, produced by a marine sponge-derived Trichoderma harzianum. Tetrahedron Lett, 2014; 55(3):662–4.
Yamada T, Suzue M, Arai T, Kikuchi T, Tanaka R. Trichodermanins C-E, new diterpenes with a fused 6-5-6-6 ring system produced by a marine sponge-derived fungus. Mar Drugs, 2017; 15(169):1–7.
Yu Z, Lang G, Kajahn I, Schmaljohann R, Imhoff JF. Scopularides A and B, cyclodepsipeptides from a marine sponge-derived fungus, Scopulariopsis brevicaulis. J Nat Prod, 2008; 71(6):1052–4.
Zhang H, Zhao Z, Wang H. Cytotoxic natural products from marine sponge-derived microorganisms. Mar Drugs, 2017; 15(68):1–13.
Zhang J, Yang Z, Liang Y, Zhong L, Lin H, Zhong B, Li L, Xu S, Liu Y. Four new C9 metabolites from the sponge-associated fungus Gliomastix sp. ZSDS1-F7-2. Mar Drugs, 2018; 16(231):1–11.
Zhao DL, Shao CL, Wang Chao Yi, Wang M, Yang LJ, Wang CY. Naphthalenones and depsidones from a sponge-derived strain of the fungus Corynespora cassiicola. Molecules, 2016a; 21(2):1–6.
Zhao HY, Anbuchezzhian R, Sun W, Shao CL, Zhang FL, Yin Y, Yu ZS, Li ZY, Wang CY. Cytotoxic nitrobenzoyloxy-substitued sesquiterpen from sponge-derived endozoic fungus Aspergilus insulicola MD10-2. Curr Pharm Biotechnol, 2016b; 17:271–4.
Zhou R, Liao X, Li H, Li J, Feng P, Zhao BX, Xu S. Isolation and synthesis of misszrtine a: a novel indole alkaloid from marine sponge-associated Aspergillus sp. SCSIO XWS03F03. Front Chem, 2018; 6(212):1–7.