INTRODUCTION
Mosquito-borne diseases are a major public health issue, including Zika virus disease, malaria, dengue fever, yellow fever, Japanese encephalitis, chikungunya, and filariasis, especially in tropical and subtropical regions, which are transmitted by the bite of an infected mosquito (Mike, 2016; World Health Organization, 2014). Globally, more than 1 million people die from mosquito-borne diseases every year, and for some diseases, the number of patients is likely to increase, such as dengue (World Health Organization, 2014). In Thailand, diseases spread by mosquitoes remain an important health concern and should be resolved urgently to reduce the losses that occur. In 2016, dengue had the highest morbidity rate of 222.58 cases per 100,000 people with dengue followed by malaria (9.11 cases per 100,000 people, Bureau of Vector Borne Disease, 2016).
Reducing the number of larvae and adult mosquito populations in nature is one of the control strategies of mosquito-borne diseases to reduce the risk of infection in communities (Roiz et al., 2012). Currently, the most common method for controlling mosquito populations in communities is the use of chemical insecticides that are inexpensive and easily available in the market. Insecticides are toxic substances that can exert adverse effects on human health and the environment (Nkya et al., 2013). For larvicide, temephos is accepted and widely used to control mosquito larvae, especially Aedes aegypti as the primary dengue vector in water containers around households. Temephos is a non-systemic organophosphorus insecticide which is not toxic to humans, plants, and animals. The World Health Organization (2009) reported that temephos was of low toxicity in rats, mice, rabbits, and guinea-pigs. As well as experiments in humans, 10 men were given temephos at a dose of 1.1 mg/kg body weight per day for 4 weeks, and 9 men took temephos at a dose of 4.27 mg/kg body weight per day for 5 days (World Health Organization, 2009). It was found that there was no inhibition of the activity of enzymes in plasma or red blood cells. Although temephos is not toxic to humans and animals, persistent use can cause mosquito resistance to insecticides (Jirakanjanakit et al., 2007). A previous study has investigated the trend of temephos resistance in Aedes mosquitoes in Thailand during 2003–2005 and found that the resistance to temephos has occurred in various parts of Thailand, including some areas in Bangkok, Nakhon Sawan (northcentral), and Nakhon Ratchasrima (northeast, Jirakanjanakit et al., 2007) which makes it difficult to control mosquito vector populations (Pimsamarn et al., 2009). At present, natural plants are used to study their effectiveness in larvae control as an alternative to temephos in some areas where resistance is found. As an adult mosquito control, mosquito traps are widely used, especially for Culex as Japanese encephalitis vectors and Anopheles as malaria vectors mosquitoes (Okumu et al., 2010). Mosquito traps must be used together with mosquito lure substances to increase the efficiency of the trap, such as octenol, carbon dioxide, and lactic acid. Usually, female mosquitoes follow these scents, which are like the smell of humans and animals because they require proteins and other nutrients from human or animal blood for egg production (Takken and Kline, 1989).
Octenol (1-octen-3-ol, also known as mushroom alcohol) is a volatile organic compound and can be found in mushrooms. It was previously reported that octenol is toxic to small insects (Inamdar and Bennett, 2014), which led to the use of mushroom extracts, including Pleurotus eryngii (Chaiphongpachara et al., 2018a), Pleurotus pulmonarius (Chaiphongpachara et al., 2018b), Auricularia auricula-judae (Chaiphongpachara et al., 2018c), Tremella fuciformis (Chaiphongpachara et al., 2018d), and Pleurotus djamor (Chaiphongpachara and Laojun, 2018) to test the killing of mosquito larvae. Although this substance is toxic to small insects, it can attract adult mosquitoes and this substance is sold in the market to be used as mosquito bait. Therefore, it is possible that some mushrooms in Thailand could kill larvae and attract adult mosquitoes as an alternative method of controlling mosquito vectors. However, octenol is very expensive and must be imported from abroad, making it unpopular in Thailand. In previous research, local edible mushroom extracts were used to test its effects in attracting mosquitoes in Samut Songkhram Province, Thailand, including Pleurotus ostreatus, Thaeogyroporus porentosus, Volvariella volvacea, Pleurotus sajor-caju, and Lentinus edodes, which was found to be slightly effective against mosquitoes in some mushrooms (Chaiphongpachara et al., 2018e).
In this study, we compared the efficacy of V. volvacea (Bull. ex Fr.) mushroom, an edible, commercially available mushroom species grown in Thailand and reported to contain octenol (Mau et al., 1997) with octenol (1-octen-3-ol) as a standard substance to kill larvae and attract adult mosquitoes. In this investigation, we examined two species of mosquito vectors, namely, A. aegypti Linnaeus as a dengue vector and Culex sitiens Wiedemann as a filariasis and Japanese encephalitis vector, both of which are major disease carriers in coastal habitats of Thailand (Chaiphongpachara and Sumruayphol, 2017). The study of the effectiveness of V. volvacea in this research may be a guideline for the development of alternative substances for mosquito control which have the advantage of being inexpensive and eco-friendly.
MATERIALS AND METHODS
Collection and identification of V. volvacea
Volvariella volvacea mushroom (also called edible straw mushroom) was collected in October 2018 at the Talat Thai market, Pathum Thani Province (Figure 1), which is a large trade source of vegetables and agricultural products in Thailand (14°4ʹ54.51″N 100°37ʹ53.06″E). Clean and fresh V. volvacea mushrooms were selected and put into storage bags for forwarding to the College of Allied Health Sciences, Suan Sunandha Rajabhat University, Samut Songkhram Provincial Education Center, immediately. After that, all the mushrooms were washed clean with filtered water, placed in the laboratory until the mushrooms dried (approximately, 1–2 hours). The type of mushroom has been identified at the collection source; however, V. volvacea was confirmed again by morphological characteristics using mushroom taxonomic identification keys (Largent, 1986; Largent and Baroni, 1988; Largent and Thiers, 1977; Stuntz, 1977).
Preparation of mushroom extract
Volvariella volvacea mushrooms were dried using sun exposure (approximately 37°C), ground into powder to increase the surface for extraction, and fermented with 95% ethanol at laboratory temperature (approximately, 25 ± 2°C) for 48 hours. Then, the obtained extract was filtered through a Whatman No. 1 paper. Ethanol was evaporated from the extracts using a rotary evaporator. After that, yields of crude extract were weighed, recorded, kept in brown bottles, and stored in a freezer at 4ºC until they were used. For testing, the crude extract of V. volvacea was dissolved in distilled water for larvicidal bioassay and by methanol for adult mosquito attraction bioassay.
Mosquito collection and rearing
Aedes aegypti Linnaeus and C. sitiens Wiedemann were used for testing in this study. Eggs of A. aegypti Bora Bora strain, which originated in Bora Bora of French Polynesia (WHO susceptible strain), were obtained from the Faculty of Tropical Medicine, Mahidol University. Culex sitiens larvae were derived from collecting in water sources of Samut Songkhram Province with a salinity level greater than 0.05 parts per thousand (ppt) by a dipper. In this study, we divided the test with mosquitoes into two parts: testing with larvae and testing with adult mosquitoes. Therefore, A. aegypti eggs and C. sitiens larvae have been reared to get the proper stage of mosquitoes for the test in larvae and adult mosquitoes. Rearing trays (25 cm × 30 cm × 5 cm) which contained filtered water were used for rearing and provided with dog food once per day. Adult mosquitoes were moved to cages (30 cm × 30 cm × 30 cm) to wait for testing.
Larvicidal bioassay
Late in the third or early fourth, instar larvae of both A. aegypti and C. sitiens were used in a larvicidal bioassay. The larval bioassay has been carried out according to the WHO guidelines (2016). Five concentrations of extract, including 120, 12, 1.2, 0.12, and 0.012 mg/l were selected to test the effectiveness of killing larvae. 0.012 mg/l has been used as the lowest concentration and increased by 10 times in the next concentration because it is the lowest concentration of larvicide susceptibility tests, as recommended by WHO (2016). The V. volvacea extracts were diluted to get the desired concentration with 100 ml of filtered water in six-ounce glasses. The larval bioassay started from the lowest concentration (0.012 mg/l) to the highest concentration (120 mg/l). Twenty healthy larvae were transferred to each glass. The number of dead larvae was counted and recorded after 24 hours to calculate the mortality. The dead larvae were observed as being non-moving and submerged. The larval bioassay has been repeated three times for each concentration. Octenol, as a comparison group (1-Octenol EMPLURA® from Merck KGaA Company, Darmstadt, Germany), has been tested in all the processes as well as mushroom extracts.
 | Figure 1. The Talat Thai market, Pathum Thani Province. [Click here to view] |
Adult mosquito attraction bioassay
Only adult female A. aegypti and C. sitiens mosquitoes were used in the adult mosquito attraction bioassay. The adult mosquito bioassay was modified from the Y-tube test according to Geier and Boeckh (1999). Three concentrations of extract, including 100, 10, and 1 mg/l were selected to test the effectiveness of adult female mosquito attraction. For choosing concentrations in this study, we have chosen concentrations of V. volvacea extract from previous research that found Aedes albopictus responds well to octanol at 10 and 1 mg/l (Guha et al., 2014). The adult mosquito bioassay started from the lowest concentration (1 mg/l) to the highest concentration (100 mg/l) and three replicates were performed for each concentration. Volvariella volvacea extracts were diluted to get the desired concentration with methanol and dripped onto Whatman filter paper. Octenol as a comparison group was diluted with methanol and dripped onto filter paper as well as V. volvacea extracts. After that, the filter paper containing V. volvacea extracts or octenol was placed at the end of the left pipe (exposure side). Filter paper containing methanol as extraction solvent (non-exposure side) was placed at the end of the right pipe. In each test, 20 healthy adult female mosquitoes were released into the entrance of the Y-tube by using a simple mouth aspirator (pooter), waited for the mosquitoes to fly to the end of the pipe, and then counted and recorded the numbers of mosquitoes in each side.
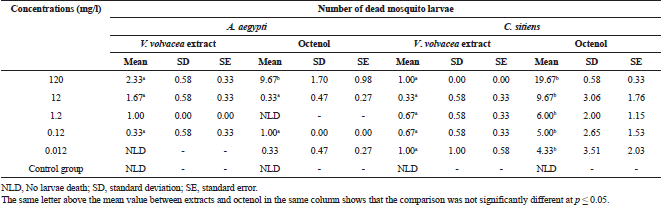 | Table 1. Statistical comparison of the number of dead larvae of V. volvacea extract and octenol at each concentration. [Click here to view] |
Statistical analyses
The numbers of dead larvae and attracted mosquitoes at each concentration were expressed as mean, standard deviation (SD), and standard error (SE). Statistical comparison of the number of dead larvae and adult female mosquitoes that respond between V. volvacea extract and octenol in each concentration was performed using the Mann–Whitney U test at a p-value of <0.05.
RESULTS AND DISCUSSION
Comparison of the larvicidal efficacy
Volvariella volvacea extract slightly killed C. sitiens larvae at all the concentrations, while A. aegypti larvae died slightly at almost all the concentrations except for 0.012 mg/l (no larvae death, Table 1). Comparing the larvicidal efficacy between V. volvacea extract and octenol at each concentration, we found that there were differences in the number of dead larvae at almost all the concentrations in testing with octenol (p < 0.05). The results of this study indicated that this mushroom extract was almost ineffective in killing larvae of both mosquito species in laboratory testing. However, octenol has shown high toxicity to C. sitiens larvae at 120 mg/l concentration. These results were consistent with previous researches that tested the activity in killing mosquito larvae of five types of mushrooms, including P. eryngii (Chaiphongpachara et al., 2018a), P. pulmonarius (Chaiphongpachara et al., 2018b), A. auricula-judae (Chaiphongpachara et al., 2018c), T. fuciformis (Chaiphongpachara et al., 2018d), and P. djamor (Chaiphongpachara and Laojun, 2018) and found that they were not effective compared with octenol. However, the effectiveness of V. volvacea extract had a slight effect on A. aegypti which was different from the five types of mushrooms in previous studies that did not kill A. aegypti.
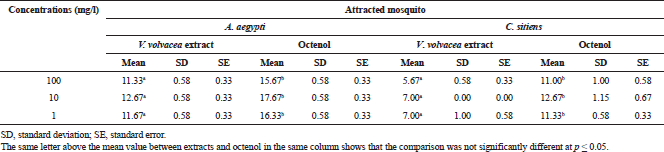 | Table 2. Statistical comparison of the mean number of adult female mosquitoes that respond between extracts and octenol at each concentration. [Click here to view] |
Comparison of the adult mosquito attraction efficacy
Volvariella volvacea extract attracted the highest number of adult female A. aegypti and C. sitiens at the 10 mg/l concentration followed by 1 and 100 mg/l, as well as the effect of octenol (Table 2). This is consistent with the results of previous research studies, which tested this substance against mosquitoes and found that 10 mg/l concentration was most effective followed by 1 mg/l (Guha et al., 2014). The numbers of A. aegypti responded to all the concentrations of extracts and octenol was clearly higher than C. sitiens. It was reported that the octenol is more effective in attracting Aedes spp. than Culex mosquitoes (Cilek et al., 2018).
The statistical comparison found that the efficiency of attracting A. aegypti and C. sitiens mosquitoes in all the concentrations of V. volvacea extract was different from octenol (p < 0.05). It is generally known that the octenol is effective in attracting adult female mosquitoes, which is often used to increase the efficiency of mosquito traps in the field. These results confirm the effectiveness of this substance in attracting mosquito vectors, including A. aegypti (>75%) and C. sitiens (>60%) in these laboratory tests. Previous research has reported that the octenol exists in this mushroom (Mau et al., 1997). Recently, V. volvacea extract has been used in combination with resting boxes to attract mosquito vectors and found to be effective in attracting mosquitoes in the field (Chaiphongpachara et al., 2018e). In this study, we found that V. volvacea extracts were less effective in attracting mosquitoes than octenol. These results are consistent with previous research studies that tested the effectiveness of five types of mushrooms in Thailand to attract mosquitoes and found that they attracted mosquitoes less than octenol.
CONCLUSION
In this study, we found that this mushroom extract was not effective in killing A. aegypti and C. sitiens larvae. However, V. volvacea extract was effective in attracting more than half the amount of A. aegypti mosquitoes in the laboratory tests but it was not equivalent to octenol.
ACKNOWLEDGMENTS
The authors would like to thank and acknowledge the College of Allied Health Science, Suan Sunandha Rajabhat University, Thailand, for support of our research.
FINANCIAL SUPPORT
None.
CONFLICT OF INTEREST
All the authors declare that they have no conflicts of interest.
REFERENCES
Bureau of Vector Borne Disease, Ministry of Public Health, Thailand. 2016. Available via http://www.thaivbd.org/n/home (Accessed 08 July 2018).
Chaiphongpachara T, Sumruayphol S. Species diversity and distribution of mosquito vectors in coastal habitats of Samut Songkhram province, Thailand. Trop Biomed, 2017; 34:524–32.
Chaiphongpachara T, Laojun S. Effect of Pleurotus djamor (Rumph. ex Fr.) Boedijn mushroom extract on larval and adult Aedes aegypti (L.) and Culex sitiens Wiedemann (Diptera : Culicidae) Mosquitoes. J Chem Pharm Sci, 2018; 11:284–7. CrossRef
Chaiphongpachara T, Bumrungsuk A, Chitsawaeng C, Sumchung K, Chansukh KK. Effectiveness of Pleurotus eryngii (king oyster mushroom) extract for killing larvae and attracting adult mosquito vectors in Samut Songkhram Province of Thailand. Biol Med, 2018a; 10:4. CrossRef
Chaiphongpachara T, Jittrabiab S, Nacapunchai D. Larvicidal and adult attractant efficiency of the edible mushroom Pleurotus pulmonarius on Aedes aegypti and Culex sitiens (Diptera, Culicidae) mosquitoes. Pak J Biotechnol, 2018b; 15:641–5.
Chaiphongpachara T, Sumchung K, Chansukh KK. Larvicidal and adult mosquito attractant activity of Auricularia auricula-judae mushroom extract on Aedes aegypti (L.) and Culex sitiens Wiedemann. J Appl Pharm Sci, 2018c; 8:21–5. CrossRef
Chaiphongpachara T, Sumchung K, Bumrungsuk A, Chansukh KK. Larvicidal and adult mosquito vector attractant activity of Tremella fuciformis Berk mushroom extract on Aedes aegypti (L.) and Culex sitiens Wiedemann (Diptera: Culicidae). J Appl Pharm Sci, 2018d; 8:7–10. CrossRef
Chaiphongpachara T, Padidpoo O, Chansukh KK, Sumruayphol S. Efficacies of five edible mushroom extracts as odor baits for resting boxes to attract mosquito vectors : a field study in Samut Songkhram Province , Thailand. Trop Biomed, 2018e; 35:653–63.
Cilek JE, Ikediobi CO, Hallmon CF, Johnson R, Onyeozili EN, Farah SM, Mazu T, Latinwo LM, Ayuk-Takem L, Berniers UR. Semi-field evaluation of several novel alkenol analogs of 1-octen-3-ol as attractants to adult Aedes albopictus and Culex quinquefasciatus. J Am Mosq Control Assoc, 2011; 27:256–62. CrossRef
Geier M, Boeckh J. A new Y-tube olfactometer for mosquitoes to measure the attractiveness of host odours. Entomol. Exp. Appl, 1999; 92:9–19. CrossRef
Guha L, Seenivasagan T, Iqbal ST, Agrawal OP, Parashar BD. Behavioral and electrophysiological responses of Aedes albopictus to certain acids and alcohols present in human skin emanations. Parasitol Res, 2014; 113:3781–7. CrossRef
Inamdar AA, Bennett JW. A common fungal volatile organic compound induces a nitric oxide mediated inflammatory response in Drosophila melanogaster. Sci Rep, 2014; 4:1–9. CrossRef
Jirakanjanakit N, Saengtharatip S, Rongnoparut P, Duchon S, Bellec C, Yoksan S. Trend of temephos resistance in Aedes (Stegomyia) mosquitoes in Thailand during 2003–2005. Environ Entomol, 2007; 36:506–11. CrossRef
Mike S. Medical entomology for students. Cambridge University Press, Liverpool, United Kingdom, 2016.
Largent DL. How to identify mushrooms to genus I: macroscopic features. Eureka Printing. Mad River Press, Eureka, CA, 1986.
Largent DL, Baroni TJ. How to identify mushrooms to genus VI: modern genera. Eureka Printing. Mad River Press, Eureka, CA, 1988.
Largent DL, Thiers HD. How to identify mushrooms to genus II: feld identifcation of genera. Eureka Printing, Eureka, CA, 1977.
Mau JL, Chyau CC, Li J. Tseng YH. Flavor compounds in straw mushrooms Volvariella volvacea harvested at different stages of maturity. J Agr Food Chem, 1997; 45:4726–9. CrossRef
Nkya TE, Akhouayri I, Kisinza W, David JP. Impact of environment on mosquito response to pyrethroid insecticides: facts, evidences and prospects. Insect Biochem Mol Biol, 2013; 43(4):407–16. CrossRef
Okumu FO, Madumla EP, John AN, Lwetoijera DW, Sumaye RD. Attracting, trapping and killing disease-transmitting mosquitoes using odor-baited stations—the Ifakara Odor-Baited Stations. Parasit Vectors, 2010; 3:12. CrossRef
Pimsamarn S, Sornpeng W, Akksilp S, Paeporn P, Limpawitthayakul M. Detection of insecticide resistance in Aedes aegypti to organophosphate and synthetic pyrethroid compounds in the north-east of Thailand. Dengue Bull, 2009; 33:194–202.
Roiz D, Roussel M, Munõz J, Ruiz S, Soriguer R, Figuerola J. Efficacy of mosquito traps for collecting potential west nile mosquito vectors in a natural mediterranean wetland. Am J Trop Med Hyg, 2012; 86:642–8. CrossRef
Takken W, Kline DL. Carbon dioxide and 1-octen-3-ol as mosquito attractants. J Am Mosq Control Assoc, 1989; 5:311–6.
Stuntz DE. How to identify mushrooms to genus IV: key to families and genera. Eureka Printing, Eureka, CA, 1977.
World Health Organization. Temephos in drinking-water: use for vector control in drinking-water sources and containers, 2009. Available via https://www.who.int/water_sanitation_health/dwq/chemicals/temephos.pdf (Accessed 08 July 2018).
World Health Organization. Vector-borne diseases, 2014. Available via http://www.who.int/kobe_centre/mediacentre/vbdfactsheet.pdf (Accessed 07 May 2018).
World Health Organization. Monitoring and managing insecticide resistance in Aedes mosquito populations Interim guidance for entomologists. WHO, Geneva, Switzerland, 2016.