INTRODUCTION
Non-alcoholic fatty liver disease (NAFLD) is globally the most common liver disease and its prevalence had progressively increased over the last decade (Dajani and Abu Hammour, 2016). NAFLD is a clinical aspect of liver damage, categorized into simple with only hepatic steatosis, and nonalcoholic steatohepatitis (NASH), where intralobular inflammation, hepatocyte ballooning degeneration, and hepatic steatosis are observed. NASH is a progressive disease and may develop into liver fibrosis, cirrhosis, and hepatocellular carcinoma (Foster et al., 2011). NAFLD/NASH is robustly linked with obesity, insulin resistance, hypertension, and dyslipidemia, and it is considered as a hepatic manifestation of the metabolic syndrome (Souza et al., 2012). Adding on, oxidative stress, as a result of an increased level of fatty acids within the hepatocytes, is thought to be responsible for the progression of NAFLD pathogenesis (Khoshbaten et al., 2010), justifying the study of several antioxidants in NAFLD treatment.
Life style modification by diet and exercise is currently the main treatment for NAFLD/NASH; however, finding of therapeutic options is requisite because NAFLD patients often have difficulty maintaining healthy life styles (Takahashi et al., 2015). Until then, treatment has focused on therapeutic medicines used for associated co-morbidities (e.g., hypertension, diabetes mellitus, hyperlipidemia, etc.), and possibly liver-protecting agents (Dajani and Abu Hammour, 2016). Although several studies revealed that treatment with atorvastatin (ATO), an inhibitor of 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase, is efficient and secure for NAFLD/NASH patients (Nseir and Mahamid, 2013); however, there are limited and conflicting reports about the statins effect on liver histology (Chalasani et al., 2012) in addition to the limited number of patients involved in these investigations. N-Acetylcysteine (NAC) is a glutathione precursor, limits the reactive oxygen species that causes hepatocellular injury and its antioxidative properties have been tested clinically and in animal models of NAFLD (Ali et al., 2016; Khoshbaten et al., 2010; Uzun et al., 2009), with encouraging results. Since NAFLD/NASH has a complicated pathogenesis, so combining agents that reduce cholesterol/load of fatty acids (e.g., ATO) with agents that reduce oxidative stress and cell injury (e.g., NAC) is reasonable, and is the best therapeutic strategy to investigate moving forward. Besides, to the best of our information, there is no report examining the ATO plus NAC combination strategy. Accordingly, the current research was conceived in rats for investigating the concomitant therapeutic impact of ATO plus NAC with and without diet control versus each drug separately on lipid profile, oxidative stress, adipocytokines, liver steatosis, inflammation, and fibrosis. Herein, we used the animal model to avoid the host confusing variables, environmental factors, including diet and lifestyle, as well as the availability and ease of histological examination.
MATERIALS AND METHODS
Animals
One hundred-eight male Sprague-Dawley rats, average weight 120 ± 20 g, were kept at the animal house of Theodor Bilharz Research Institute (TBRI), Giza, Egypt. Rats were accommodated in individual cages under controlled temperature (25°C) and 12/12-hour light/dark cycle, with ad libitum access to food and water, 1 week before initiation of the experiment. All the animal experiments were carried out according to the international guidelines for the care and use of laboratory animals and were approved by the Institutional Review Board of TBRI (TBRI-IRB/FWA00010609/Feb. 2018).
Experimental design
Nine groups, each of 12 rats, were randomly classified into: normal control, rats fed on high-fat diet (NAFLD-HFD model), HFD-rats switched to regular diet (NAFLD-RD model), NAFLD-HFD or -RD rats treated with ATO, NAC or the combined ATO + NAC, respectively. Rats in the control group were maintained on the standard chow diet for 20 weeks. NAFLD-rats were induced by feeding a HFD of [25% fats + 1% cholesterol (Win lab Laboratory chemicals) + 0.25% bile salts (Alpha chemika, India)] for 12 weeks according to Zulet et al. (1999), with slight modification. After 12 weeks, rats in the ATO/NAC-treated NAFLD groups were treated with ATO (Ator®; Egyptian International Pharmaceutical Industries Company, EIPICO, 10th of Ramadan City, Egypt) at a dose of 30 mg/kg/day b.w. (Roglans et al., 2002) or NAC (Sigma-Aldrich Chemical Company, St. Louis, MO) at a dose of 500 mg/kg/day b.w. (Samuhasaneeto et al., 2007) via oral gavage, for 8 weeks (starting from the 13th week to the 20th week). Rats in the NAFLD-RD treated groups were switched and maintained on the standard chow diet (weeks 13–20). At the end of experiment and after overnight fasting, rats of all the groups were weighted and then killed with an intraperitoneal injection of ketamine (80 mg/kg). Blood samples were collected and sera were separated for biochemical assessment, and the livers were immediately removed, weighted, and sampled for assay of oxidative stress markers, and for histopathological examination. Liver weight index (%) = Liver weight/body weight × 100.
Study of biochemical markers
Liver function tests, including serum alanine and aspartate transaminases (ALT and AST), gamma glutamyl transferase (γ-GT), and alkaline phosphatase (ALP), as well as the lipid profile; total cholesterol (TC), triglycerides (TG), and high-density lipoprotein (HDL) were assayed spectrophotometrically using the available commercial kits. Low-density lipoprotein (LDL) was calculated using Friedewald formula: [LDL = TC − HDL − TG/5 (mg/dl)]. Very low-density lipoprotein (VLDL) was calculated by formula [VLDL = TC − (HDL + LDL) (mg/dl)]. Serum transforming growth factor-β1 (TGF-β1; IBL International GMBH, Hamburg, Germany), tumor necrosis factor-α (TNF-α; Assaypro, St. Louis, MO), adiponectin (RayBiotech Inc., Norcross, GA), and leptin (Assaypro, St. Louis, MO) levels were measured using ELISA kits.
Study of lipid peroxidation and oxidative stress
Liver samples (1 gm each) were homogenized (Pro Scientific Inc., U.S.A. homogenizer) in ice-cold 100 mM KH2PO4 buffer (1:4 w/v; pH 7.4) containing 1 mM Ethylenediaminetetraacetic acid (EDTA) and centrifuged at 10,000 g for one hour at 4°C. Aliquots of the collected supernatant were used for subsequent analysis of reduced glutathione (GSH) content, superoxide dismutase (SOD) activity and degree of liver lipid peroxidation [expressed by malondialdehyde (MDA) formation] spectrophotometrically using the available commercial kits.
Histological examinations
Specimens of liver tissues were fixed in 10% buffered formaldehyde, embedded in paraffin wax, then sectioned (5 μm thick) and stained with hematoxylin-eosin (H&E). Steatosis was examined morphologically by semi-quantitative analysis, at high power microscopic fields of ×400/section, using the computerized image analysis system (Axiovision version 4.8, Zeiss Germany), and graded as the following: mild = 5%−30%, moderate = 30%−60%, and severe >60% of hepatocytes affected (Kleiner et al., 2005). Also, histological changes, such as ballooning degeneration, vacuolation, acidophilic necrosis, sinusoidal fibrosis, and polymorph nuclear infiltration were examined.
Statistical analysis
Data are expressed as the mean ± SEM and analyzed using the one-way ANOVA test followed by Tukey’s post hoc test (SPSS, software package version 16.0, Chicago, IL). Data are considered statistically significant if p < 0.05.
RESULTS
Hepatic gross manifestations
The body, liver weights, and liver index in the NAFLD-rats were increased significantly when compared to the corresponding values of normal controls. Compared with the NAFLD untreated group, a significant decrease (p < 0.05) in body, liver weights, and index was observed in rats treated with ATO alone or ATO + NAC with the shift of data toward normal values in these parameters in groups treated with both drugs weather maintained on HFD or RD (Table 1).
Biochemical and oxidative stress markers
Following treatment of HFD-group with ATO for 8 weeks with or without reverting to RD, serum lipids (Table 2), liver enzymes (Fig. 1), and adipocytokines (Fig. 2) were significantly improved (p < 0.05) as compared with the parallel-untreated groups. Moreover, GSH, SOD, and MDA were significantly ameliorated when ATO treated group reverted into RD (Fig. 3). Significant improvements (p < 0.05) in TC, TG, LDL (Table 2), ALT, AST, γ-GT (Fig. 1), adiponectin, leptin, TNF-α, and TGF-β (Fig. 2) were observed in the group treated with NAC alone. In addition, administration of NAC alone or in combination with ATO significantly decreased the effects of the oxidative stress and lipid peroxidation by restoring GSH content, increasing SOD activity and decreasing of MDA level (Fig. 3). In the ATO/NAC combined groups, most of the measured serum and hepatic markers gave better and significant results over those of each individual-treated group, weather maintained on HFD or switched to RD.
Histology
After 20 weeks, in the normal control group (Fig. 4A), the liver lobules were intact with preserved lobular architecture, the liver cell cords arranged regularly with no fat aggregation, or inflammatory infiltration, or hepatocyte ballooning. The liver in NAFLD/HFD model group showed notable steatosis with abundant fat deposition (Fig. 4B), steatosis was predominantly macrovesicular (up to 65% of the lobules), with isolated single droplets and nuclear eccentricity; nevertheless, microvesicular steatosis were also noted in some hepatocytes. Hepatocytes ballooning degeneration, as a result of accumulation of intracellular fluid, was characterized by swollen cells. Based on the observations of the number of lymphocytes, moderate inflammation was found in the lobular and portal areas with moderate fibrosis in the form of thin fibrous bands (Fig. 4B); however, in NAFLD/RD, steatosis involved up to 37% of the lobules (Fig. 4C) with moderate inflammation. The degree of hepatic damage as manifested by steatosis, inflammatory infiltration, hepatocyte ballooning, and fibrosis were moderately alleviated in ATO group (Fig. 4D and E) and partially improved in NAC group (Fig. 4F and G). In the ATO + NAC treated group, steatosis and lobular inflammation were mitigated significantly over those of each drug separately, and in all NAFLD-RD groups more than those groups maintained on HFD (Fig. 4H and I) where steatosis (<20%) and extension of lobular inflammation were below the lower limit for the diagnosis of steatohepatitis (Table 3). In addition, all the treated groups showed that lipid deposits were smaller than untreated groups, and no signs of cirrhosis were observed in all examined groups (Fig. 4A−I).
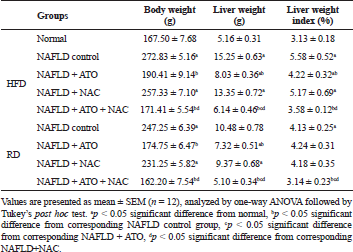 | Table 1. Effect of atorvastatin and N-acetylcysteine alone or in combination on liver gross manifestations in non-alcoholic fatty liver disease-induced rats. [Click here to view] |
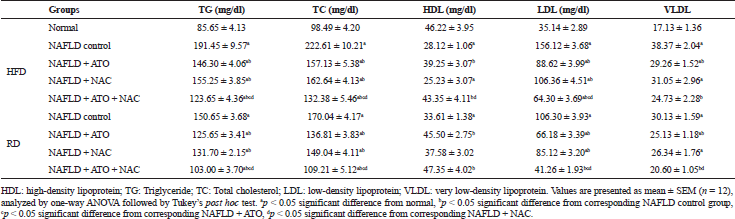 | Table 2. Effect of atorvastatin and N-acetylcysteine alone or in combination on lipid profile in non-alcoholic fatty liver disease-induced rats. [Click here to view] |
DISCUSSION
The data of this study showed that HFD aggravated blood liver enzymes, lipid profile, inflammatory, and oxidative stress markers in rats. Moreover, the histological profile showed liver steatosis, lobular inflammatory infiltrates, hepatocytes ballooning, and fibrosis. In agreement with a previous study (Hernández-Guerra et al., 2006), ATO significantly increased HDL, lowered TC, TG, LDL, and VLDL. As a non-HDL cholesterol-decreasing agent, ATO catalyzes the conversion of HMG-CoA into mevalonate, an important step in the cholesterol biosynthesis, leading to lower liver cholesterol production, high plasmatic clearance of LDL cholesterol and up-regulation of hepatocyte LDL receptors (Taylor et al., 2011). The current study also revealed that NAC significantly lowered TC, TG, and LDL. Similar results were obtained in mice where treatment with NAC almost completely blocked lipid accumulation in the liver, which was evidenced by lower liver levels of triglyceride and cholesterol (Ma et al., 2016).
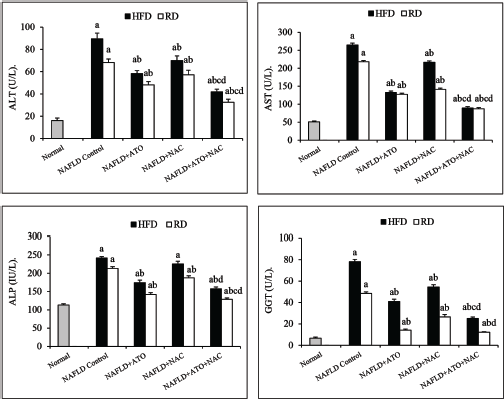 | Figure 1. Effect of atorvastatin and N-acetylcysteine alone or in combination on serum liver enzymes in NAFLD-induced rats. Values are presented as mean ± SEM (n = 12). ap < 0.05 versus normal, bp < 0.05 versus corresponding NAFLD control group, cp < 0.05 versus NAFLD + ATO-treated group, dp < 0.05 versus NAFLD+NAC-treated group. [Click here to view] |
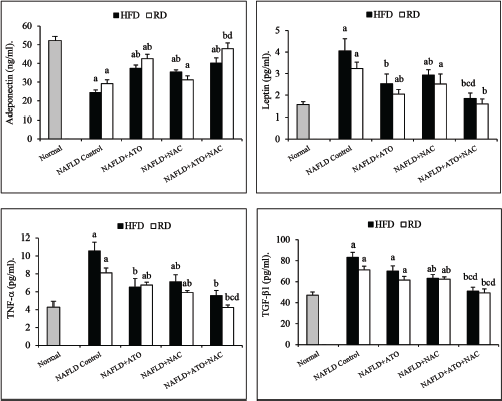 | Figure 2. Effect of atorvastatin and N-acetylcysteine alone or in combination on adipocytokine markers in NAFLD-induced rats. Values are presented as mean ± SEM (n = 12). ap < 0.05 versus normal, bp < 0.05 versus corresponding NAFLD control group, cp < 0.05 versus NAFLD + ATO-treated group, dp < 0.05 versus NAFLD + NAC-treated group. [Click here to view] |
.png) | Figure 3. Effect of atorvastatin and N-acetylcysteine alone or in combination on oxidative stress markers and lipid peroxidation in NAFLD-induced rats. Values are presented as mean ± SEM, (n = 12). aP < 0.05 versus normal, bP < 0.05 versus corresponding NAFLD control group, cP < 0.05 versus NAFLD+ATO-treated group, dP < 0.05 versus NAFLD+NAC-treated group. [Click here to view] |
Reactive oxygen species as a result of oxidative stress have contributed to impaired liver cellular function leading to cell death and necrosis (Stärkel et al., 2003). This impaired cellular function could be monitored by determining serum transaminases (ALT and AST) which are considered among the most sensitive biochemical markers investigated in the diagnosis of liver diseases and the best indicators of liver necrosis (Amacher et al., 2013). In this study, the elevated transaminase serum levels in HFD rats reflect the hepatocyte damage that was investigated histopathologically in this study. Eight weeks after the treatment with either ATO or NAC alone or both, these abnormalities were significantly improved, suggesting that ATO and/or NAC could alleviate the HFD-induced liver injury. This favorable impact on serum transaminases could reflect on the amelioration of liver histopathology and less fatty infiltration in hepatocytes as shown in the present investigation.
It is assumed that adipokines and cytokines, such as adiponectin, leptin, and TNF-α, have an important role in pathogenesis and severity of NAFLD (Giby and Ajith, 2014). The effect of HFD on leptin and adiponectin level in this study was in accordance with Ali et al. (2016), who reported that leptin levels were significantly higher in NAFLD/NASH rats; meanwhile, adiponectin levels were significantly lower when compared to controls. In disagreement with the results of Mäuser et al. (2007) who mentioned that ATO decreased adiponectin synthesis in mature adipocytes, data of this study showed that ATO partially restored the depleted adiponectin levels in HFD group; meanwhile, it significantly lowered the leptin level. The same findings were recorded in groups treated with NAC. NAC was able to reduce high levels of leptin while increasing adiponectin levels in female mice prenatally exposed to a HFD (Berry et al., 2018). Also, it could improve the obesity-related disturbed adipocytokine metabolism through alleviating the TNF-α-induced oxidant-antioxidant imbalance in adipocytes (Araki et al., 2006). In spite of several small, short term, and uncontrolled trials on statins have documented a reduction in hepatic steatosis, the majority of these did not give a report on liver histology; however, there is agreement that statins do not have a positive impact on liver histology in NASH but can be safely prescribed to mend their increased cardiovascular risk (Chalasani et al., 2012). In this study, ATO alone was not able to significantly reduce the hepatic oxidative stress indicators. Nevertheless, histopathology examination displayed that ATO might decrease significantly both steatosis (~23% vs. 65% for HFD-group and 13% vs. 37% for HFD-RD group) and liver portal inflammation as shown by diminution in the number of inflammatory foci and their density. Adding on, ATO could markedly attenuate the serum TNF-α with partial decline in TGF-β. In agreement with our study, Martın-Castillo et al. (2010) reported that ATO therapy decreased TC and TG, liver steatosis, inflammation, and hepatocellular damage in hyperlipidemic chickens. The function of statins in mitigating inflammation is assumed to be as a result of suppression of neutrophils chemotaxis and reduction of pro-inflammatory cytokines (Dunzendorfer et al., 1997) or through lowering both stress-activated c-Jun N-terminal kinase (JNK) activation and the hepatic expression of TGF-β (Wang et al., 2013).
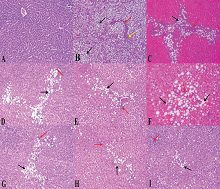 | Figure 4. Liver sections showing: (A) normal control, intact hepatic lobular architecture and normal morphological appearance; (B) NAFLD/HFD, partial disturbed hepatic lobular architecture, severe micro and macro steatotic changes about 80% (black arrow), fibrous bands porto-portal and porto-central (red arrow) and moderate interlobular inflammatory cells (yellow arrow); (C) NAFLD/RD, moderate micro and macro steatotic changes (black arrow), mild interlobular inflammation (red arrow); (D) ATO/HFD, mild micro and macro steatotic changes (black arrow), mild interlobular inflammation (red arrow); (E) ATO/RD, improved steatotic changes (black arrow) and mild interlobular inflammation (red arrow); (F) NAC/HFD, mild−moderate micro and macro steatotic changes (black arrow), moderate interlobular inflammation (red arrow); (G) NAC/RD, mild micro steatotic changes (black arrow) and mild interlobular inflammation (red arrow); (H) ATO + NAC/HFD, scattered micro steatotic changes (black arrow) and mild interlobular inflammation (red arrow); and (I) ATO + NAC/RD, enhancement improvements in steatotic changes (black arrow) and interlobular inflammation (red arrow) and almost normal morphological appearance. Mild = 5%−30%, moderate = 30%−60%, and severe >60% of hepatocytes affected. The hematoxylin and eosin images were captured under magnification ×200. [Click here to view] |
In the current study, NAC markedly recovered the reduction in hepatic GSH, abolished lipid peroxidation, and suppressed TNF-α, TGF-β as well as the inflammation, resulting in a significant decline in cellular damage, hepatocyte injury, and fibrosis as displayed histopathologically. These amendments are in agreement with those previously reported by Baumgardner et al. (2008). Oxidative stress, regardless of its etiology, plays a major role in hepatic fibrogenesis (Tsukamoto, 1993). Previous studies reported that NAC blocked experimental fibrosis/cirrhosis through abolishing oxidative stress and decreasing TGF-β (Pereira-Filho et al., 2008; Galicia-Moreno et al., 2009). Herein, the first report on ATO and NAC combination therapy showed enhanced ameliorations in hepatic inflammation, fibrosis, and oxidative stress markers over each drug separately. Furthermore, they alleviated hepatic steatosis, concomitant with modulation of liver function, and lipid profile, leading to a reduction in the severity of hyperlipidemia in NAFLD rats. These dual effects were more manifested under dietary control and this confirms that diet control and personal lifestyle adjustments are crucial for the control of hyperlipidemia. These results strengthen the utility of this combination therapy in modulating metabolic profile and protecting liver in hyperlipidemic rats.
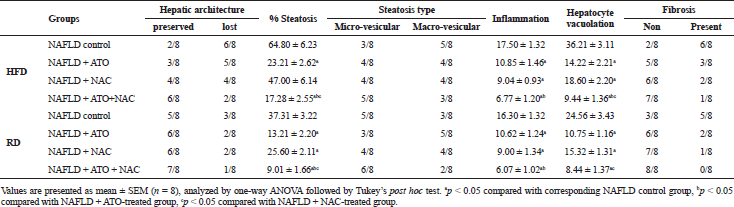 | Table 3. Effect of atorvastatin and N-acetylcysteine alone or in combination on liver histological parameters in non-alcoholic fatty liver disease-induced rats. [Click here to view] |
CONCLUSION
The current study demonstrated that the diet control is still the first intervention for NAFLD treatment. Combination of ATO with NAC under diet control has the added benefits on reducing the lipid levels, liver enzymes, oxidative stress markers, hepatic steatosis, inflammation, and fibrosis versus each drug separately. Accordingly, this combination strategy is recommended for further investigation in clinical trials.
ACKNOWLEDGMENT
This study is a part from a research project (grant No. 95/A) for basic and applied research and was financially supported by Theodor Bilharz Research Institute.
CONFLICT OF INTEREST
No conflict of interest.
REFERENCES
Ali MH, Messiha BA, Abdel-Latif, HA. Protective effect of ursodeoxycholic acid, resveratrol, and N-acetylcysteine on nonalcoholic fatty liver disease in rats. Pharm Biol, 2016; 54:1198−208. CrossRef
Amacher DE, Schomaker SJ, Aubrecht J. Development of blood biomarkers for drug-induced liver injury: an evaluation of their potential for risk assessment and diagnostics. Mol Diagn Ther, 2013; 17:343−54. CrossRef
Araki S, Dobashi K, Kubo K, Yamamoto Y, Asayama K, Shirahata A. N-acetylcysteine attenuates TNF-alpha induced changes in secretion ofinterleukin-6, plasminogen activator inhibitor-1 and adiponectin from 3T3-L1adipocytes. Life Sci, 2006; 79:2405−12. CrossRef
Baumgardner JN, Shankar K, Hennings L, Badger TM, Ronis MJ. A new model for non-alcoholic steatohepatitis in the rat utilizing total enteral nutrition to overfeed a high polyunsaturated fat diet. Am J Physiol Gastrointest Liver Physiol, 2008; 294:G27−38. CrossRef
Berry A, Bellisario V, Panetta P, Raggi C, Magnifico MC, Arese M, Cirulli F. Administration of the antioxidant N-acetylcysteine in pregnant mice has long-term positive effects on metabolic and behavioral endpoints of male and female offspring prenatally exposed to a high-fat diet. Front Behav Neurosci, 2018; 12:48. CrossRef
Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, Charlton M, Sanyal AJ. The diagnosis and management of nonalcoholic fatty liver disease: practice guideline by the American Gastroenterological Association, American Association for the Study of Liver Diseases, and American College of Gastroenterology. Gastroenterology, 2012; 142:1592−609. CrossRef
Dajani A, Abu Hammour A. Treatment of nonalcoholic fatty liver disease: where do we Stand? An Overview. Saudi J Gastroenterol, 2016; 22:91−104.
Dunzendorfer S, Rothbucher D, Schratzberger P, Reinisch N, Kahler CM, Wiedermann CJ. Mevelonate-dependent inhibition of transendothelial migration and chemotaxis of human peripheral blood neutrophils by pravastatin. Circ Res, 1997; 81:963−69. CrossRef
Foster T, Matthew J, Budoff SS, Naser A, Craig G, Alan D, Guerci MD. Atorvastatin and antioxidants for the treatment of nonalcoholic fatty liver disease: the St Francis Heart Study Randomized Clinical Trial. Am J Gastroenterol, 2011; 106:71−7.
Galicia-Moreno M, Rodríguez-Rivera A, Reyes-Gordillo K, Segovia J, Shibayama M, Tsutsumi V, Vergara P, Moreno MG, Muriel P. N-acetylcysteine prevents carbon tetrachloride-induced liver cirrhosis: role of liver transforming growth factor-beta and oxidative stress. Eur J Gastroenterol Hepatol, 2009; 21:908−14. CrossRef
Giby VG, Ajith TA. Role of adipokines and peroxisome proliferator-activated receptors in nonalcoholic fatty liver disease. World J Hepatol, 2014; 6:570−79. CrossRef
Hernández-Guerra M, García-Pagán JC, Turnes J, Bellot P, Deulofeu R, Abraldes JG, Bosch J. Ascorbic acid improves the intrahepatic endothelial dysfunction of patients with cirrhosis and portal hypertension. Hepatology, 2006; 43:485−91. CrossRef
Khoshbaten M, Aliasgarzadeh A, Masnadi K, Tarzamani MK, Farhang S, Babaei H, Kiani J, Zaare M, Najafipoor F. N-acetylcysteine improves liver function in patients with non-alcoholic Fatty liver disease. Hepat Mon, 2010; 10:12−6. CrossRef
Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS, Unalp-Arida A, Yeh M, McCullough AJ, Sanyal AJ; Nonalcoholic Steatohepatitis Clinical Research Network. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology, 2005; 41:1313−21. CrossRef
Ma Y, Gao M, Liu D. N-acetylcysteine protects mice from high fat diet-induced metabolic disorders. Pharm Res, 2016; 33:2033−42. CrossRef
Martın-Castillo A, Castells MT, Ada´nez G, Sa´nchez Polo, MT Pe´rez BG, Ayala I. Effect of atorvastatin and diet on non-alcoholic fatty liver disease activity score in hyperlipidemic chickens. Biomed Pharmacother, 2010; 64:275−81. CrossRef
Mäuser W, Perwitz N, Meier B, Fasshauer M, Klein J. Direct adipotropic actions of atorvastatin: differentiation state-dependent induction of apoptosis, modulation of endocrine function, and inhibition of glucose uptake. Eur J Pharmacol, 2007; 564:37−46. CrossRef
Nseir W, Mahamid M. Statins in nonalcoholic fatty liver disease and steatohepatitis: updated review. Curr Atheroscler Rep, 2013; 15:305. CrossRef
Pereira-Filho G, Ferreira C, Schwengber A, Marroni C, Zettler C, Marroni N. Role of N-acetylcysteine on fibrosis and oxidative stress in cirrhotic rats. Arq Gastroenterol, 2008; 45:156−62. CrossRef
Roglans N, Sanguino E, Peris C, Alegret M, Vázquez M, Adzet T, Díaz C, Hernández G, Laguna JC, Sánchez RM. Atorvastatin treatment induced peroxisome proliferator-activated receptor alpha expression and decreased plasma non esterified fatty acids and liver triglyceride in fructose-fed rats. J Pharmacol Exp Ther, 2002; 302:232−39. CrossRef
Samuhasaneeto S, Thong-Ngam D, Kulaputana O, Patumraj S, Klaikeaw N. Effects of N-acetylcysteine on oxidative stress in rats with non-alcoholic steatohepatitis. J Med Assoc Thai, 2007; 90:788−97.
Souza MR, DinizMde F, Medeiros-Filho JE, Araújo MS. Metabolic syndrome an drisk factors for non-alcoholic fatty liver disease. Arq de Gastroenterol, 2012; 49:89−96. CrossRef
Stärkel P, Sempoux C, Leclercq I, Herin M, Deby C, Desager JP, Horsmans Y. Oxidative stress, KLF6 and transforming growth factor-beta up-regulation differentiate non-alcoholic steatohepatitis progressing to fibrosis from uncomplicated steatosis in rats. J Hepatol, 2003; 39:538−46. CrossRef
Takahashi Y, Sugimoto K, Inui H, Fukusato T. Current pharmacological therapies for nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. World J Gastroenterol, 2015; 21:3777−85. CrossRef
Taylor F, Huffman MD, Macedo AF, Moore TH, Burke M, Davey Smith G, Ward K, Ebrahim S. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev, 2011; 19:CD004816. CrossRef
Tsukamoto H. Oxidative stress, antioxidants, and alcoholic liver fibrogenesis. Alcohol, 1993; 10:465−7. CrossRef
Uzun MA, Koksal N, Kadioglu H, Gunerhan Y, Aktas S, Dursun N, Sehirli AO. Effects of N-acetylcysteine on regeneration following partial hepatectomy in rats with nonalcoholic fatty liver disease. Surg Today, 2009; 39:592−97. CrossRef
Wang W, Zhao C, Zhou J, Zhen Z, Wang Y, Shen C. Simvastatin ameliorates liver fibrosis via mediating nitric oxide synthase in rats with non-alcoholic steatohepatitis related liver fibrosis. PLoS One, 2013; 8:e76538. CrossRef
Zulet MA, Barber A, Garcin H, Higueret P, Martinez JA. Alterations in carbohydrate and lipid metabolism induced by a diet rich in coconut oil and cholesterol in a rat model. J Am Coll Nutr, 1999; 1:3642. CrossRef