INTRODUCTION
Gemfibrozil [5-(2, 5- dimethylphenoxy)-2, 2- dimethyl-pentatonic acid] is an antilipemic agent similar to clofibrate, which is a derivate of fibric acid. It is used in the treatment of hyperlipoproteinemia and a second-line drug in type IIb hypercholesterolemia. Gemfibrozil is commercially available as film-coated tablets and capsules (600-mg tablets and 300-mg capsules). Extrahepatic lipoprotein lipase activity was increased by gemfibrozil. It will lead to increase the lipoprotein triglyceride lipolysis, thereby activating peroxisome proliferator activated receptor-alpha and this receptor will involve in the metabolism process of carbohydrates, fats, and adipose tissue differentiation. The t1/2 of gemfibrozil was 1.5 hours, and more than 95% of the drug has affinity toward the plasma (Drug information, 2018).
The literature survey shows a few high performance liquid chromatography (HPLC) methods for the determination of gemfibrozil in human plasma (Gonzaález-Pen~as et al., 2000; Kim et al., 2007; Rao et al., 2012 ; Kang et al., 2009; Rower et al., 2010; Vittal et al., 2006) and to the best of our knowledge, an analytical method for gemfibrozil using HPLC has been reported (Sushma et al., 2013 ; Ulu, 2006) in which the method had low sensitivity and selectivity (LOD and LOQ). Furthermore, the developed method had long run time with complicated gradient elution. Hence, the present study is aimed to develop a simple, sensitive, and selective economical mass spectrometric method for the quantification of Gemfibrozil active pharmaceutical ingredient (API).
MATERIALS AND METHODS
Chemicals
Working standard Gemfibrozil was obtained from Ranbaxy Laboratories Ltd as a gift sample, LC-MS/MS grade methanol was procured from Sigma Aldrich, and Ammonium formate and formic acid were procured from Rankem Fine Limited. Used water of LC-MS grade was procured from Millipore, Bedford, USA.
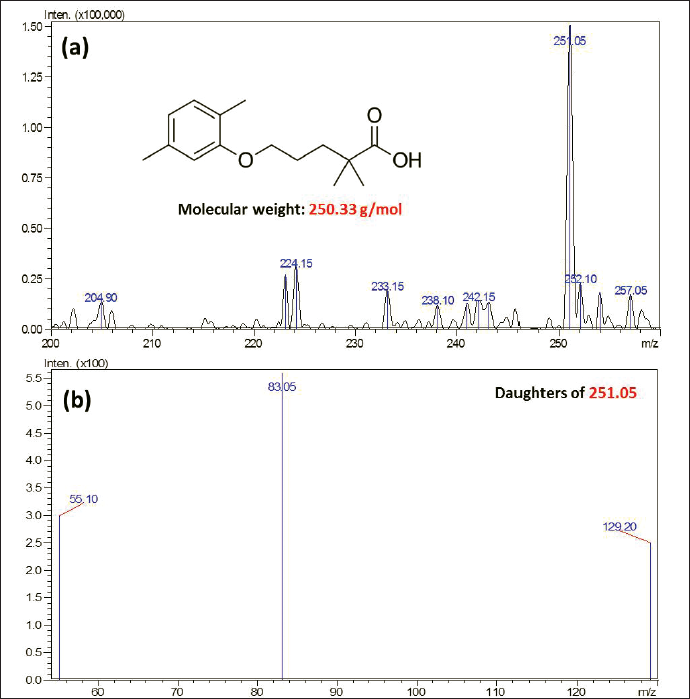 | Figure 1. (a) Scan mass spectra for Gemfibrozil in positive ionization and (b) MS/MS spectra for Gemfibrozil. [Click here to view] |
Sample preparation
Preparation of working standard solution
Ten milligram of Gemfibrozil was dissolved in 10 ml of solvent (methanol) to produce 10 mg/ml solution. Working concentration of 1 μg/ml solution was prepared from 10 mg/ml of above given solution.
Selection of a mass range
For optimizing the operation conditions, 1,000 ng/ml of Gemfibrozil solution was injected directly into the mass spectrometer. Obtained transitions were 251.15 → 83.05 (CE ‒17.0) and 251.15 → 128.95 (CE ‒12.0) used to monitor the Gemfibrozil (Fig. 1).
Equipment and chromatographic conditions
A triple quadrupole mass spectrometer coupled with LC system equipped with electrospray ionization interface, LC-20AD pump, CTO-20AC column oven, SIL-20AC autosampler, and CBM-20 alite controller was used. Lab solution data station software was used for recording the data. Zorbax SB C18 column (4.6 × 50 mm, 3 μm) was used as stationary phase with mobile phase consist of a mixture of (10 mM) ammonium formate (pH 3.5): methanol (20:80 V/V). The flow rate was 0.8 ml/minute and the injection volume of 10 μl was employed (Fig. 2).
Method validation
The method was validated for accuracy, precision, specificity, linearity, range, and detection limit, quantitation limit, robustness, and system suitability as per ICH guidelines (ICH, 1996).
Accuracy
According to ICH guidelines, recovery studies were done to determine the accuracy of the method. The drug gemfibrozil was spiked with the pre-analysed samples.
Precision
Inter-day and intra-day studies were carried out for evaluation of precision. Studied samples were studied at three concentration levels (n = 6) of low quality control (LQC, 1 ng/ml), middle quality control (MQC, 7.5 ng/ml) and high quality control, (HQC, 40 ng/ml), respectively. Regressed concentration of the percent coefficient variance (% CV) was used for precision report.
Specificity
The ability to quantify the analyte in the presence of other impurities can be termed as specificity.
Linearity
For the linearity range covered from 1 to 50 ng/ml (n = 6) for six concentration levels covering for Gemfibrozil, the evaluation of linearity was performed. By using a calibration curve calculation of the coefficient correlation, slope and intercept values were identified for linearity evaluation.
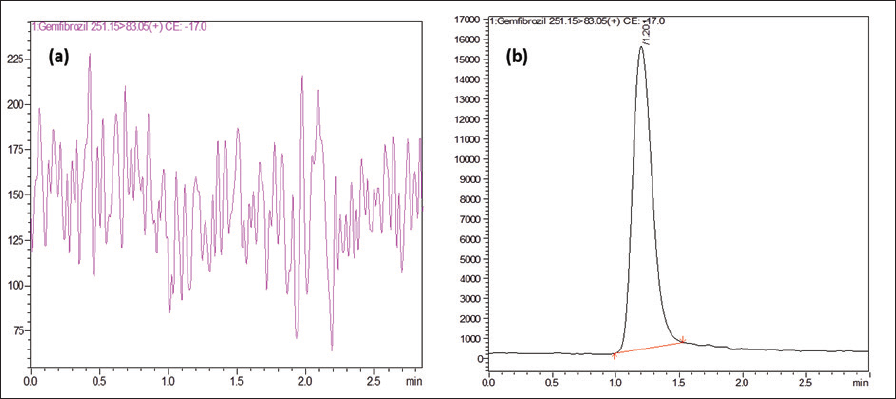 | Figure 2. (a) Blank chromatogram for gemfibrozil. (b) Standard chromatogram of Gemfibrozil. [Click here to view] |
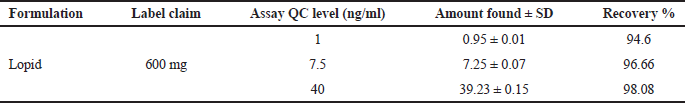 | Table 1. Recovery studies for formulation. [Click here to view] |
Determination of Gemfibrozil dosage form
Twenty tablets were weighed and powdered for the estimation of Gemfibrozil from the tablets and weight equivalent to 100 mg was weighed accurately and transferred to a 100-ml volumetric flask. The content was dissolved using 50-ml solvent and filtered through a 0.45-μm filter and the final volume was made up to mark with solvent methanol and the further diluted to produce the quality control sample of low-quality control 1.0 ng/ml, medium quality control 7.5 ng/ml, and high-quality control 40 ng/ml. Table 1 summarizes the recovery results of Gemfibrozil in the formulation (Fig. 3).
Detection (LOD) and quantitation limit (LOQ)
Detection and quantitation limit was performed based on the signal to noise ratio (S/N) with minimum peak area for LOD (3:1) and LOQ (10:1).
Robustness
For evaluating the robustness of the method the like operators, the source of reagents, similar type column, and optimized conditions like pH, mobile phase ratio, and the flow rate were studied.
System suitability
Determining the system suitability is the integral part for method development process which is determined for the number of theoretical plates (N) and tailing factor (T).
RESULTS AND DISCUSSIONS
Accuracy
By standard addition, the accuracy of the method was carried out for three concentrations (LQC, MQC, and HQC) samples, and the accuracy was found to be 94.03%–100.02%. The developed method can be used for the quantification of Gemfibrozil in commercial formulation (Table 2). The intraday accuracy values for LQC (1 ng/ml), MQC (7.5 ng/ml), and HQC (40 ng/ml) were found to be 96.00, 97.33, and 99.01, respectively. The inter-day accuracy was found to be 94.60, 96.66, and 98.08; compared to the previous studies, the developed method shows high accuracy and precision.
Precision
Precision studies were calculated for inter-day and intra-day studies at three different concentrations and they were found to be within the limits. The intraday % CV values for LQC (1 ng/ml), MQC (7.5 ng/ml), and HQC (40 ng/ml) were found to be 1.04, 1.36, and 0.14, respectively. The inter-day % CV was found to be 1.61, 0.97, and 0.38.
Specificity
The developed method was selective for the determination of Gemfibrozil in the formulation as no peaks were eluted along with the drug (Fig. 2a). The developed method showed a short retention time of 1.201 minutes compared to the previously reported studies and the reported studies were not found to be reproducible due to its complicated gradient method.
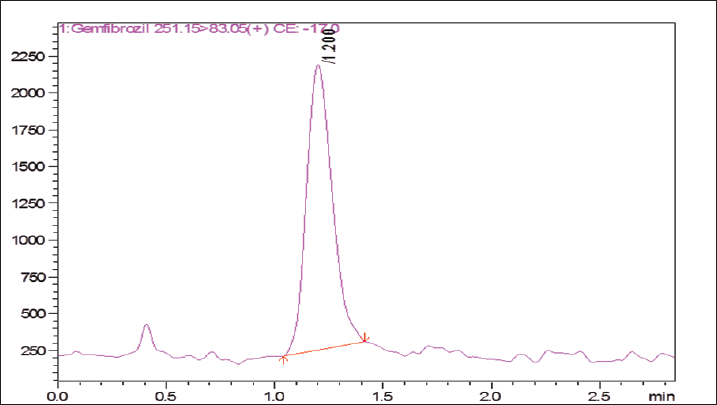 | Figure 3. Multiple reaction monitoring chromatogram of Gemfibrozil (Formulation). [Click here to view] |
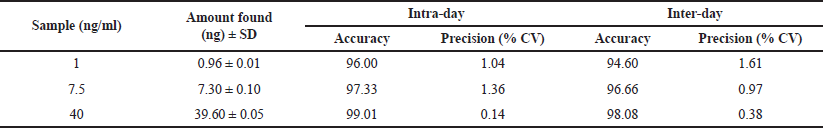 | Table 2. Determination of accuracy and precision studies for Gemfibrozil. [Click here to view] |
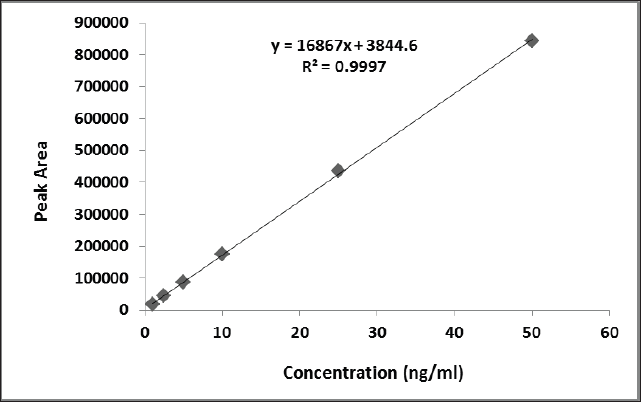 | Figure 4. Calibration curve of Gemfibrozil. [Click here to view] |
Calibration curve
The method was evaluated to be linear based on the regression equation with r2 0.999 for Gemfibrozil and the standard deviation (SD) was found to be within the limits. A calibration curve was found to be linear with a mean regression of equation Y = 16867x + 3844.6, respectively, where the analyte peak area is Y and the analyte peak area concentration in ng/ml is X (Fig. 4).
Robustness
By altering the chromatographic conditions, the robustness of the method was determined and the results were found to be robust. The conditions such as, pH, flow rate (ml/min), and % methanol were used for the robustness testing. Robustness studies were carried out for three levels at six replicates. pH of the mobile phase was adjusted to 3, 3.5, and 4. The % CV was found to be 0.10, 0.12, and 0.12. Flow rate was adjusted to 0.5, 0.8, and 1 ml/minute and the % CV was found to be 0.21, 0.05, and 0.103. Percentage methanol was adjusted to the following ratios of 70%, 80%, 90% and % CV was found to be within the limits. The proposed method was more accurate and stable while altering the conditions compared to previously reported studies.
The previously reported method for the quantification of gemfibrozil in pharmaceutical formulation using RP-HPLC methods was found to be not much sensitive for the detection of gemfibrozil in pharmaceutical formulation due to their detection and quantitation limit, whereas in the present method gemfibrozil was detected and quantified at 0.5 and 1.0 ng/ml, respectively, which indicated that this method was sensitive and can detect and quantify lower level of gemfibrozil in pharmaceutical formulation. The linearity range was from 1 to 50 ng/ml with a regression coefficient of 0.999 indicating this concentration range of gemfibrozil was highly linear.
CONCLUSION
The developed liquid chromatography-tandem mass spectroscopy method was found to be novel, simple, accurate, and precise. The developed method can be successfully applied for the estimation of gemfibrozil in the marketed formulations and in active pharmaceutical ingredient. All the parameters and results were found within the acceptance limits as given in the validation protocol.
ACKNOWLEDGMENTS
The authors are grateful to the Ranbaxy Laboratories Ltd for providing the standard gemfibrozil as a gift sample.
CONFLICT OF INTEREST
All the authors declare that there are no conflicts of interest.
FINANCIAL SUPPORT AND SPONSORSHIP
None.
REFERENCES
Drug information. [Online]. Available via http://www.drugs.com (Accessed 10 October 2018).
Gonzaález-Pen~as E, Agarraberes S, Ocariz AL, Quetglas EG, Campanero MA, Carballal JJ, Honorato J. A sensitive method for the determination of gemfibrozil in human plasma samples by RP-LC. J Pharm Biomed Anal, 2001; 26:7–14. CrossRef
ICH. Q3B validation of analytical procedures: methodology, International Conference on Harmonization. November 1996 [Online]. Available via https://www.fda.gov/downloads/drugs/guidances/ucm073384.pdf (Accessed 20 October 2018).
Kang X, Wang F, Xie Z, Li H. A high performance liquid chromatography method for simultaneous determination of rosiglitazone and gemfibrozil in human plasma. J Chromatogr B, 2009; 877(7):645–8. CrossRef
Kim CK, Jae JP, Hwang HR, Ban E, Maeng JE, Kim MK, Piao XL. Simple and sensitive HPLC method for determination of gemfibrozil in human plasma with fluorescence detection. J Liq Chromatogr Rel Tech, 2006; 29(3):403–14. CrossRef
Rao KN, Satyanarayanaa PVV, Suneethaa G, Venkateswarlu P. Development and validation of sensitive RP- HPLC method for determination of gemfibrozil in human plasma. Der Pharma Chemica, 2012; 4(3):882–8.
Rower JE, Bushman LR, Hammond KP, Kadam RS, Aquilante CL. Validation of an LC/MS method for the determination of gemfibrozil in human plasma and its application to a pharmacokinetic study. Biomed Chromatogr, 2010; 24(12):1300–8. CrossRef
Sushma A, Harinadha Babu K, Vijay Kumar G. New analytical method development and validation for the estimation of gemfibrozil in bulk and pharmaceutical formulation by RP-HPLC. Int J Pharm, 2013; 3(1):173–5.
Ulu ST. LC determination of gemfibrozil in tablets. Chromatographia, 2006; 64. CrossRef
Vittal S, Shitut NR, Kumar TR, Vinu MC, Mullangi R, Srinivas NR. Simultaneous quantitation of rosuvastatin and gemfibrozil in human plasma by high-performance liquid chromatography and its application to a pharmacokinetic study. Biomed Chromatogr, 2006; 20(11):1252–9. CrossRef