INTRODUCTION
Rheumatoid arthritis (RA) an autoimmune disease due to which there is loss of joint space leading to its dysfunctionality and ultimately deformity. It is progressive in nature and causes irreversible damage to the synovial-lined joints. It has widespread presence of about 1% in adult population. The prevalence of RA in common population has been estimated to be 0.8%, especially begins with fourth and fifth decades of life with a two- to three-fold greater frequency in women than in men (rheumatology.org; Rudan et al., 2015). It has been estimated that by 2030, 41 million of the world population aged 65 and older will have arthritis (Herman et al., 2004). The risk of disability can be high as 33% and due to the result of infection or circulatory disease the mortality rate can be up to 52% of the total population effected and it has a considerable impact on the standard of life (Schwartzman et al., 2004). Despite an increased understanding of the pathophysiology of RA, there are no treatments that cure RA. Therefore, the therapeutic goals are to minimize symptoms, regain complete functionality and to minimize further remission with disease-modifying anti-rheumatic drug (DMARD) therapy (O’Dell, 2004).
At present non-steroidal anti-inflammatory drugs, steroids and DMARDs are used for the management of RA. The use of these drugs is also limiting due to their major side effects and their tendency for causing adverse effects such as stomach ulcers, thrombocytopenia, and pulmonary toxicity, and prolong use may lead to increase in risk of stroke and heart attack (Garner et al., 2014).
Methotrexate (MTX), generally regarded as a “gold standard for treatment of RA,” is the most used DMARDs (Shinde et al., 2014). Though we are aware that RA is an autoimmune disease, exact etiology of RA is still unknown (Chauhan et al., 2018). The oral administration of MTX in low weekly doses is effective in reducing inflammation in RA (Sharma and Arora, 2012). Oral utilization has indicated systemic toxicity. Patients who require initial high dose of MTX have low tolerance, though within the therapeutic range when administered orally (Lima et al., 2015; Vena et al., 2018). As per the available literature, patients prescribed with MTX do show systemic toxicity over prolonged use. However, these effects are observed in conventional dosage forms (tablets, capsules and injections) where the entire dose was presented after the administration. MTX toxicity develops due to increased patient susceptibility during the treatment, excessive parenteral or intrathecal administration, therapeutic errors by patients (e.g., taking MTX orally daily instead of weekly), self-administration, or intentional oral overdoses. One of the possible methods of reducing the toxicity could be limiting the exposure of MTX unless required to elicit the pharmacological response. In this regard, one of the aims of our study was to reduce the possible toxicity of MTX by delivering in a sustained manner. Therefore, previous attempts have been made to enhance the efficacy and lower the adverse drug reactions of MTX by developing formulation for intra articular route of administration. These results were ineffective since MTX was rapidly eliminated from the intra articular cavity (Wigginton et al., 1980).
Intra articular injection of a controlled release drug delivery system has been proposed in order to maintain an effective dose of the drug in the joint cavity and reduce the drug related toxicity by minimizing exposure to body tissues. This system is considered to be more advantageous over conventional delivery such as that via oral, intravenous, or intramuscular routes since the release of the drug can be prolonged by altering the specification, such as method of formulation, shape and size, polymer type, chemical nature, molecular weight of the polymer, etc. which in turn limits the systemic side effect. Studies have demonstrated that hydrogel can retard the drug (MTX) metabolism and removal from joint cavity while releasing the drug in controlled manner (Bolong et al., 2011; Linda et al., 2004).
Injectable in situ gels offer many advantages over conventional oral dosage forms. They can be easily formulated, sterilized, and delivered locally or at specific site in the body. These systems reduce dose, dosing frequency, associated side effects, cost of therapy and thereby improving patient compliance. In situ gels exhibit phase transition from sol to gel due to physiological change in the environment. They can be easily delivered subcutaneously or into the synovial cavity. At physiological conditions (37°C), forms a depot releasing drug in a predictable and controlled manner (Venkatesh et al., 2011). This delivery system has longer retention time and can easily be administered into the body.
MATERIALS AND METHODS
Materials
MTX was supplied by Strides Arcolabs Limited, Bengaluru; Pluronic F-127 (Sigma Aldrich, USA), xanthan gum (CP Kelco, USA), Benzalkonium Chloride (Reachem Labs, Chennai), Potassium Dihydrogen Phosphate (Merck Specialities Ltd, Mumbai), Hydrochloric Acid (Reachem Lab, Chennai), Sodium Hydroxide (Merck Specialities Ltd, Mumbai), LA395 Dialysis Membrane 135 (Hi-media labs, Mumbai) were procured and used without any further processing.
Method
Preparation of MTX in situ gels
In situ gels of MTX were prepared by “Cold technique” as previously described by Schmolka (1972). MTX in situ gels were formulated using PF-127 as thermosensitive polymer and xanthan gum of concentration, 0.2%–0.6%, was incorporated in the formulation as co-polymer.
Xanthan gum was dissolved in water at 60°C and allowed to cool to room temperature. Further processing was carried after the solution attained 25°C ± 2°C. A weighed amount of PF-127 was gradually added to the cold solution (5°C–10°C) of xanthan gum under magnetic stirring. The container was sealed and left overnight in a refrigerator at 5°C until a clear solution was obtained. MTX was dissolved in 0.1-N NaOH and added dropwise to the preformed gel under continuous manual stirring, to obtain the final concentration. The pH of the final formulation was adjusted to neutral using dilute solution of Triethanolamine. Benzalkonium Chloride (0.001% w/v) was used as a preservative. The prepared gels were transferred in to 10-ml borosil glass vials and sealed. They were refrigerated at 2°C–8°C until further use. The formulation chart is given in Table 1.
Sterilization of MTX in situ gels
The sterilization of the final formulations filled in glass vials, sealed in a nitrogen atmosphere was carried out by exposing to gamma rays from a Cobalt-60 chamber at 15.76 kGy dose at MICROTROL’s Gamma Radiation Services facility, Bengaluru.
Characterization
Fourier transform infrared spectroscopy (FT-IR)
Pure drug and optimized formulations were analyzed by KBr pellet method using FT-IR spectrophotometer (Shimadzu-8400S, Japan). The samples were scanned from 400 to 4,000 cm−1 wave number. The obtained spectra were analyzed for any possible interactions with drug and polymers.
Evaluation
Microbiological evaluation
The efficiency of the method employed for sterilizing MTX in situ gels was evaluated by incubating the sterile in situ gels in fluid thioglycollate media for aerobic and anaerobic bacteria at 37°C ± 1°C and soybean casein digest media for fungal organisms at 25°C for a period of 14 days. At the end of incubation period, the growth of bacteria/fungus was observed.
Gelation temperature
Evaluation of gelation temperature was done by placing a thin walled tube (containing 2 ml of MTX in situ gel) in thermostatically regulated water bath and the temperature of which was increased at consistent rate of 2°C/5 minutes with gentle shaking at periodic intervals till it was transformed into the gel. The temperature (°C) at which it was distinguished from “flow liquid sol” to “no flow solid gel” upon the inversion of tube was considered to be the gelation temperature of the sample (Martin and Bjoern, 1992; Siriporn et al., 1997).
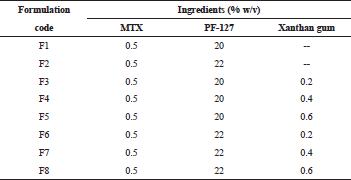 | Table 1. Formulation chart of MTX in situ gels. [Click here to view] |
Gelation time
Gelation time is defined as the time taken for sol to gel conversion when placed at gelation temperature. Test tube inverting technique was used to determine the gelation time which involves the use of thin-walled glass tube (containing 2 ml of MTX in situ gel) placed in thermostatically controlled water bath at the gelation temperature (previously determined) with gentle shaking at periodic intervals. The time (second) required for conversion to gel is recorded as gelation time which was evaluated by flow or no-flow criterion when the test tube was inverted (Sun, 2009).
Uniformity of drug content
Phosphate buffer of pH 7.4 was used to dissolve 1 ml of in situ gel and volume was made up to 10 ml. Drug content was deduced using UV-visible spectrophotometer (Shimadzu-1800S, Japan) at wavelength 304 nm after suitable dilution.
Syringeability test
Syringeability test was performed by a method described by Yannic (2008). Syringeability of gels were evaluated with a device composed of vertical support for a 5.0-ml glass syringe filled with in situ gel maintained at 5°C ± 1°C held by a vertical support with a pan placed on the piston of the syringe. The syringe was fixed with 18G needle and a mass of 0.5 kg was placed on the pan; the time (second) required for the gel to be removed entirely from the syringe was considered as syringeability time.
Viscosity study
Brookfield viscometer (DV-II+, Brookfield, USA) was used to carry out the viscosity measurements with suitable spindle (no. 8) at suitable speed (30 rpm) (Venkatesh et al., 2013). Thermostated water jacket was utilized to record viscosity (n = 3) at two temperatures 8°C ± 1°C and at 37°C ± 1°C. The samples were equilibrated for 10 minutes before measurement; also, the instrument was equipped with a temperature control unit.
In vitro drug release studies
Static diffusion method
The dialysis membrane (DM-135, Hi-Media, Mumbai) was used for diffusion studies. The hydrated membrane soaked in diffusion medium was used. One milliliter of gel was placed in dialysis membrane sac (area approx 1.4 cm2) and was sealed on both ends. The dialysis membrane was then placed in a glass beaker containing diffusion medium containing 30 ml of pH 7.4 buffer solution. The drug release study was carried out at 37°C ± 0.5°C. At regular intervals a known volume of sample was withdrawn and replaced with equal volume of fresh warm buffer solution. Drug concentration was determined using UV-Visible spectrophotometer (Shimadzu-1800, Japan) at 304 nm. Furthermore, release data were analysed by means of diverse mathematical models to know release kinetics (Zaki Rizkalla et al., 2011).
Dynamic diffusion method
The hydrated dialysis membrane (DM-135, Hi-Media, Mumbai) was tied to one end of the open (diffusion) tube and slightly immersed in the receptor medium comprising of 30 ml of pH 7.4 phosphate buffer in 100 ml Borosil beaker; 1 ml of in situ gel was placed inside dialysis membrane, which acted as the donor compartment. The receptor medium was maintained at 37 C ± 0.5°C, stirred at 100 rpm. At regular intervals, a 3 ml of diffusion medium was withdrawn from the receptor compartment and replaced with equal volume of fresh warm buffer solution. The drug concentration was determined using UV-Visible spectrophotometer (Shimadzu-1800S, Japan) at wavelength 304 nm (Venkatesh et al., 2013).
In vivo studies
Ethical approval (JSSCP Mysuru No. 130/2013) was obtained from Institutional Animal Ethics Committee JSSCP, SS Nagar, Mysuru, India for conducting animal experimentation. A total of 12 Male Wistar rats (200 ± 20 g, six in each group) were used in adjuvant arthritis study.
On day “0”, induction of adjuvant disease was done with injecting 0.1 ml of 1% solution of Freund’s reagent intraplantarly. The paw volumes were measured by plethysmometer (Ugo basile, 37140) on alternate days till day 14 to assess the development of arthritis in animals. On day 14, the animals were randomized in different treatment group viz. control and test group and treatment was started on day 14. Test group was administered with acacia daily and intra-articular administration of 100 µl MTX in situ gel once a week for 2 weeks till day 28. The control group was administered orally with acacia daily and 100 µl saline intrarticularly once a week till day 28 in left knee. Paw volume was measured every second day till day 28. After measurement of paw volume on day 28, the paw from the rest of the animals were dissected out, tissue was sliced from arthritic paw and analyzed for histopathological studies.
Histopathology
Knee joints of the sacrificed rats were separated and placed in 10% neutral buffered formalin for 24 hours and later transferred into decalcifying solution (HCl and 0.1-M Ethylenediaminetetraacetic acid) for approximately 1 week. Paraffin embedment was done for the knee joints. Fine sections were cut from the paraffin cubes and stained with hematoxylin and eosin. Furthermore, it was examined for histopathology and photographed at ×40 magnification under the microscope.
Evaluation of biocompatibility
The MTX in situ gel biocompatibility with the tissue upon injection was evaluated by histopathological studies in male Wistar rats (200 ± 20 g), 100 µl of the saline solution was injected into the left knee joint as control and in the right knee joint, 100 µl of optimized MTX in situ gel was injected. Till the seventh day, the swelling of the joints was monitored by plethysmometer, then the rats were sacrificed and joints were isolated for further study. Hematoxylin and eosin staining was used to assess cell infiltration at 40× magnification under the microscope.
Stability studies
Stability studies were conducted as per ICH guidelines (Q1A R2). Optimized in situ gels were packed in the glass vials fixed with rubber bungs with aluminum crimping; study was conducted by placing the samples in stability chamber (Thermolab Stability Chamber, Thermo lab, 2010) at 5°C ± 2°C and 25°C ± 2°C/60% ± 5% RH for 3 months.
RESULTS AND DISCUSSION
In situ gels of MTX were prepared by “Cold technique” as previously described. MTX in situ gels were formulated using PF-127 (20% and 22%) as thermosensitive polymer and xanthan gum (0.2%–0.6%) and characterization were carried out to assess their properties.
Microbiological evaluation (Sterility testing)
MTX in situ gels are meant to be injected into the body. Hence, it is desirable that the formulation should be sterile. The in situ gels were sterilized using gamma rays radiations since autoclaving may affect the physical characteristics of gel and its reversible thermogelation properties. Sterility testing was done to ascertain the effectiveness of sterilization. At the end of study period (14 days), there was no growth of aerobic and anaerobic bacteria observed in fluid thioglycollate media. Similarly, no growth was observed in the soyabean casein digest media. The results indicated that the prepared in situ gels were sterile and the method used for sterilization was effective.
Fourier transform infrared spectroscopy
MTX and the in situ gel formulation (F8) were analyzed for compatibility testing by FT-IR to ascertain any interactions between the polymers and the drug used. The FT-IR spectra of MTX, xanthan gum, pluronic, and its physical mixture were determined. The functional groups with corresponding peaks of pure MTX and in physical mixture of MTX and polymers were found to be correlative. The prominent peaks of MTX, i.e., 3,363.97 cm−1 to N-H stretching; 1,645.33 cm−1 to C=O stretch; 1,448.59 cm−1 to CH deformation (CH3); and 831.35 cm−1 to C-H deformation (aromatic) were found in the FT-IR spectra of physical mixture as shown in Figure 1. The FT-IR spectra of the pure drug, with other co-polymers showed all the characteristic peaks of MTX and there was no change in their position, indicating no chemical interactions between them. These results confirmed the compatibility of drug and polymer/s used.
Thermosensitivity characterization
Gelation temperature
The gelation temperature of MTX in situ gels ranged from 29.6°C ± 0.23°C to 38.3°C ± 0.42°C. Out of eight in situ gels, two formulations (F1 and F3) gelled at a temperature little above 37°C. Formulation F8 exhibited the lowest gelation temperature of 29.6°C ± 0.23°C and F1 the highest gelation temperature of 38.3°C ± 0.42°C (Table 2). All other formulations got transformed to gel below the body temperature. The presence of co-polymer significantly altered the process of PF-127 gelation process. In the study, incorporation of xanthan gum (0.2%–0.6% w/v) showed noticeable effect on the gelation temperature, which was concentration dependant.
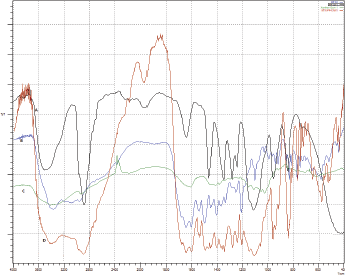 | Figure 1. FT-IR spectra of MTX (A), Xanthan gum (B), PF 127(C), and Physical Mixture (D). [Click here to view] |
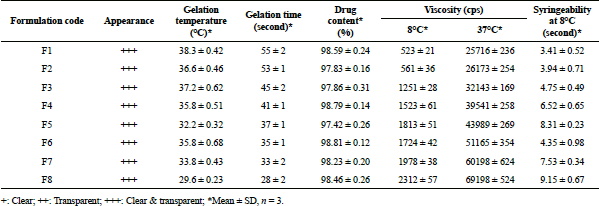 | Table 2. Characteristics of prepared MTX in situ gels. [Click here to view] |
Gelation of PF-127 occurs due to dehydration of the PF-127; pluronics get dehydrated leading to increased chain friction and enlargement, creating a hydrophobic circle. Micelles are formed in aqueous pluronic solutions. At higher temperature or at high concentrations, these micelles associate to form various lyotropic isotropic liquid crystalline phases. With the increase in temperature, micellar entanglement proceeds, leading to formation of gel and resulting in the overall increase of bulk viscosity. With increase in length of poly (oxyethylene) (PEO) chain the onset and temperature of gelation and thermal stability of the gel also increases (Attwood et al., 1985; Miller and Drabik, 1984; Paavola et al., 1998; Singhare et al., 2005) (Fig. 2).
Gelation time
When the solutions were heated in thermostatically controlled water bath, they transformed into non-flowing gels within a short period of time (<55 seconds for all formulations). At low temperature, poly(ethyleneoxide–b-propylene oxide–b-ethylene oxide) (PEO-b-PPO-b-PEO) molecules are surrounded by a hydration layer but as the temperature increases, there is a breakage of the hydrogen bond between the hydrophilic chain and solvents. This results in hydrophobic interactions within polyoxypropylene domains and leads to the formation of gel. The gelation time for MTX gels was between 28 ± 2 seconds and 55 ± 2 seconds. As observed, gelation time decreased as the concentration of the co-polymers was increased in the formulation. Longest gelation time of 55 ± 1 seconds was recorded with F1, whereas shortest gelation time of 28 ± 2 seconds was with F8 (Table 2). A decrease of 8 seconds was observed with increase of xanthan gum concentration from 0.2% to 0.6% in formulations F3 to F5. Quick in situ gelation helps in retaining the drug at the site of injection and release in a controlled manner.
Uniformity of drug content
The results of drug content in the gel sample showed proper distribution of MTX in prepared in situ gels. The drug content values ranged from 97.83% ± 0.18% to 98.81% ± 0.20% w/v (Table 2), this implied that the drug distribution was uniform and satisfactory.
Viscosity studies
The viscosity studies of in situ gels showed a temperature dependent increase in viscosity. Increase in the micelle concentration due to the shortening of the intermicellar distance causes increase in the viscosity of the in situ gel. The viscosity of gels was recorded at 8°C ± 1°C and was found in the range of 523–2,312 cps. When the same formulations were studied for viscosity at 37°C, a substantial increase in viscosity was observed; the viscosity values of 25,716–69,198 cps were recorded. As noticed from Table 2, formulations F1 and F8 exhibited the lowest (523 cps) and the highest (2,312 cps) values, respectively, at 8°C. At 37°C, F1 and F8 exhibited the lowest (25,716 cps) and the highest (69,198 cps) viscosity values, respectively (Table 2). A concentration dependent increase in viscosity was observed in the presence of co-polymers. Viscosity of in situ gels increased from 51,165 to 69,198 cps in the presence of xanthan gum in formulations F6–F8 (0.2%–0.6%). Xanthan gum chains at the room temperature are highly extended and forms helix structures by hydrogen bonding due to the electrostatic repulsion of the charged groups. As the temperature is increased, the long chains of xanthan forms a coil like conformation. In case of the in situ gels, the long chains of xanthan gum penetrate between different copolymer micelles and due to the interactions with PEO corona, they assure a higher elasticity and resulting in high viscosity. The presence of co-polymer may have elevated the viscosity of smart gels (Bercea et al., 2013).
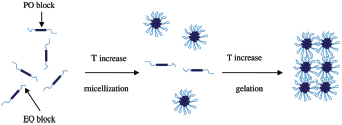 | Figure 2. Schematic representation of gelation mechanism of PF-127 in water. [Click here to view] |
Syringeability test
Syringeability test of the prepared in situ gel were carried out to determine the force required to inject the in situ gel and its effect upon change in the temperature. During administration of the in situ gels into the body at the desired site using a syringe and needle, syringeability plays an important role. Syringeability includes factors such as, accuracy of dose measurements, ease of withdrawal, and followed by pressure or force required for injection and freedom from clogging. The ease of withdrawal of a product from a container and its subsequent injection to the site of action must be determined and characterized during formulation development. In the present study, all formulations were easily syringeable.
Syringeability study was carried out on the samples kept at a refrigerated temperature of 8°C at which viscosity of in situ gels was found to be in the range of 523–2,312 cps. Administration of injectable preparations which are viscous or suspensions into the body is done by 18 guage needles to prevent the clogging of needle during injection; the prepared in situ gel systems were easily syringeable through syringe and needle (no. 18). The recorded syringeability time of the prepared in situ gel was found to be between 3.41 ± 0.52 seconds to 9.15 ± 0.67 seconds. It was observed that syringeability time increased with increase in viscosity. Formulation F1 recorded the lowest syringeability time of 3.41 ± 0.52 seconds and formulation F8 with 9.15 ± 0.67 seconds, the highest (Table 2). The results of this study implied that all the formulations were syringeable through 18 gauge needle.
In vitro drug release study
The results obtained from in vitro diffusion studies inferred that drug release was in a sustained manner (Figs. 3 and 4). Gelation of PF-127 occurs as a result of dehydration of the polymer, the Pluronic micelles come in contact with one another causing the entanglements among the hydrophilic corona of PEO chains, and thus the gel structure is formed. Due to the increase in the temperature, it can form hexagonal-packed cylindrical micelles, which stack together, leading to decreased release rates (Oh et al., 2004).
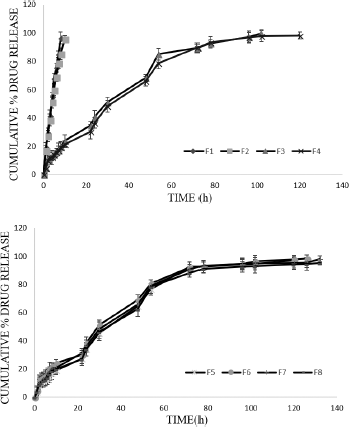 | Figure 3. In vitro drug release profile of MTX in situ gels by static method. [Click here to view] |
Due to the decrease in the intermicellar distance, there were a greater number of cross-links between neighboring micelles which ultimately resulted in higher viscosity that in turn retarded the drug release (Garala, 2013). Literature review has suggested methods for assessing the drug release of the gels for control release. In this study, mainly two methods used in this study, i.e., dynamic diffusion and static diffusion. Many scientific literatures published details of dynamic diffusion conditions, despite some proposed static conditions to be the best suited method to predict the drug release and its bioavailability (Singhare et al., 2005). A rational approach which imitates in vivo conditions must be utilized. Since in situ gels are administered parenterally, both static and dynamic conditions prevail at the site of injection. During the study, both static method and dynamic methods were used to assess the impact of stirring on in vitro drug release. Irrespective of the method of diffusion employed, all the formulations (except F1 and F2) released MTX in a controlled manner. In the most of the cases, the drug release was sustained for 102–132 hours. The concentration of polymer and co-polymer affected the drug release from the MTX in situ gels. The drug release was faster with the formulations containing lower concentration of polymer/co-polymer compared to formulations with higher concentration of polymer/co-polymer.
Method I (Static diffusion method)
At the end of the diffusion studies, F8 released was about 95.29% ± 1.12% at 132 hours and F3 recorded a release of 99.76% ± 2.67% at the end of 102 hours. Rest of the formulations (F4–F7) also displayed sustained drug release from 102 to 132 hours. The in vitro release data for static method is graphically represented in Figure 3.
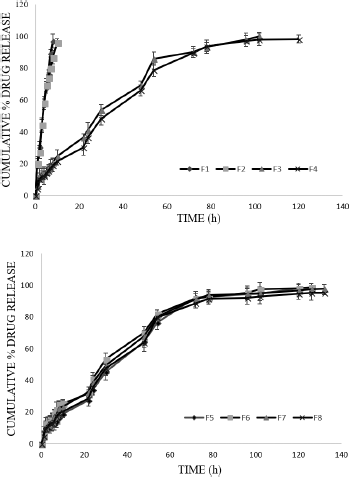 | Figure 4. In vitro drug release profile of MTX in situ gels by dynamic method. [Click here to view] |
Formulations F1 and F2 with PF-127 showed drug release up to 10 hours. Drug release was retarded from 10 hours to 102–132 hours due to incorporated co-polymer in the gels. Formulation F8 (95.29% ± 1.12% at the end of 132 hours) was considered better than rest of the formulations.
Method II (Dynamic diffusion method)
A similar trend (like static diffusion) was observed in dynamic diffusion conditions because of polymer and co-polymer. Formulation F3 showed the maximum release of 99.73% ± 2.67% (at 102th hour). F8 formulation showed 95.79% ± 4.22% at the end of 132 hours. The in vitro release data for dynamic method is graphically represented in Figure 4.
It was noticed that the formulation F1 and F2 showed high release (burst) of drug during initial period followed by slow controlled release as diffusion continued in both diffusion conditions. The high release may be due to leaching of extra micellar drug present in the aqueous channels that may be released before the complete gelation occurred.
F8 was taken as the optimized formulation as it retarded the drug release for 132 hours with the suitable gelation time and temperature compared to other formulations.
 | Table 3. Data of various parameters of model fitting for MTX in situ gel. [Click here to view] |
Mathematical model fitting
The data obtained form in vitro drug release study by static diffusion (Method I) were assessed by various mathematical models to get best fit model and to determine the release mechanism. The results of kinetic study revealed that the drug release from all formulations were controlled and followed first-order kinetics (concentration dependant) and the best fit model was Peppas model.
The drug release data (<70%) were fit in Korsmeyer Peppas equation; “n” values for all formulations was in between 0.6352 and 0.7994, The n value for Korsmeyer–Peppas model was seen to be in the range between 0.45 and 0.89, which is indicative of non-Fickian diffusion. The values obtained for the optimized formulation in mathematical model fitting are presented in Table 3.
In vivo studies
A total of 12 male Wistar rats (200 ± 20 g, six in each group) were used in the study. During the inducement stage, the paw volumes increased gradually after injecting 1% Freund’s reagent till day 14; which confirmed the development of arthritis. After inducing arthritis, treatment with optimized formulation F8 MTX in situ gels continued for the following 14 days.
Paw volume was measured every alternate day till day 28. There was a decrease in the edema from the day 17 and a noticeable decrease in the paw volume by the end of the treatment (Fig. 5).
At the end of the study period (day 28), left paw of all the rats (both control and test group) were dissected. The tissue from arthritic paw was extracted for histopathological studies.
Histopathological studies clearly differentiated the presence of inflammatory cells in both test and control groups. The inflammatory cells in test group were less in number compared to control group; which had higher inflamed cells in articular bone tissue and extra articular tissue (Fig. 6). This can be attributed to the therapeutic effect of MTX in situ gels on Wistar rats which decreased the paw volume.
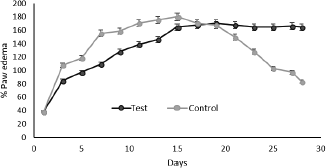 | Figure 5. Percentage paw edema of test and control. [Click here to view] |
Evaluation of biocompatibility
The test animals did not show any macroscopic signs of knee/tissue swelling nor joint stiffness. Histopathology of rats injected with MTX in situ gels or saline in joints are shown in Figure 7. No inflammation was seen in synovial membrane in both test and control sites (joints). The results demonstrate the biocompatibility of the MTX in situ gels and their suitability for intra articular drug delivery.
Stability studies
The formulation F8 fulfilled the desired characteristics; short gelation temperature and time, easy syringeability, greater viscosity which leads to reduce in the friction and also acts as lubricating gel at the joint, uniform drug content, and sustained in vitro drug release, and was tested for stability as per ICH guidelines.
The stability studies were conducted at 5°C ± 3°C and at 25°C/60% RH for 3 months. There was no marked change in the physical properties; gelation temperature and time, and drug content during the study period, which indicated that F8 formulation exhibited good stability during investigation period (Table 4).
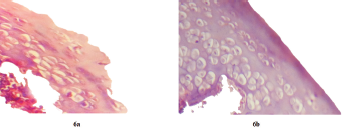 | Figure 6. Histopathologic changes observed in left knee joints of adjuvant control (6a) and adjuvant test (6b) (magnification: ×40). [Click here to view] |
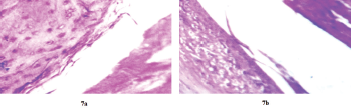 | Figure 7. Histological slides of synovial tissues after intra articular injection of saline (7a) and MTX in situ gel (7b) (magnification: ×40). [Click here to view] |
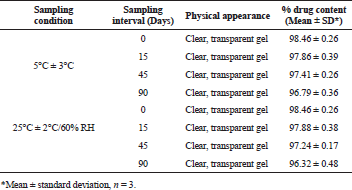 | Table 4. Stability data of MTX in situ gels. [Click here to view] |
CONCLUSION
In the present research, a sterile, thermosensitive, stable, biocompatible, injectable gel capable of releasing MTX at a controlled rate was formulated. The MTX release followed non-fickian diffusion and was concentration dependant; affected by the nature and concentration of polymer and copolymers. An improvement in inflammatory condition and no signs of articular destruction were observed in test when compared with control group during histopathological studies. The prepared MTX in situ gels proved to be promising drug delivery system which can reduce the dose, dosing frequency, and envisaged as an effective intra-articular drug delivery for the management of RA.
ACKNOWLEDGMENTS
The authors sincerely thank Dr. T. M. Pramod, Principal, JSS College of Pharmacy, Mysuru, for his support and encouragement. Our gratitude goes to JSS Academy of Higher Education & Research, Mysuru for providing all the necessary facilities.
CONFLICT OF INTEREST
The authors confirm no conflict of interest for this manuscript.
FINANCIAL SUPPORT
None.
ETHICAL APPROVAL
All experimental procedures were performed in accordance with the ethical guidelines for the study and was approved by the Institutional Animal Ethical Committee (IAEC), JSS College of Pharmacy, JSS Academy of Higher Education & Research, Mysuru, India.
REFERENCES
Attwood D, Collet JH, Tait C. The micellar properties of the poly(oxyethylene)– poly(oxypropylene) copolymer Pluronic F- 127 in water and electrolyte solution. Int J Pharm, 1985; 26:25–33. CrossRef
American College of Rheumatology. Rheumatoid arthritis [homepage on the Internet]. Available via http://www.rheumatology.org/practice/clinical/patients/diseasesandconditions/ra.asp (Accessed 15 March 2019).
Bolong M, Cunxian S, Guilei M. Injectable thermosensitive hydrogels for intra-articular delivery of methotrexate. J Appl Polym Sci, 2011; 122:2139–45. CrossRef
Bercea M, Darie RN, Morariu S. Rheological investigation of xanthan/Pluronic F127 hydrogels. Rev Roum Chim, 2013; 58:189–96.
Chauhan K, Al-Dhahir MA. Arthritis, Rheumatoid. InStatPearls Publishing, St. Petersburg, FL, 2018.
Garala K, Joshi P, Shah M, Ramkishan A, Patel J. Formulation and evaluation of periodontal in situ gel. Int J Pharm Invest, 2013; 3:29. CrossRef
Garner R, Ding T, Deighton C. Management of rheumatoid arthritis. Medicine, 2014; 42:237–42. CrossRef
Herman CJ, Allen P, Hunt WC, Prasad A, Brady TJ. Use of complementary therapies among primary care clinic patients with arthritis. Prev Chronic Dis, 2004; 1:1–12.
Lima A, Sousa H, Monteiro J, Azevedo R, Medeiros R, Seabra V. Genetic polymorphisms in low-dose methotrexate transporters: current relevance as methotrexate therapeutic outcome biomarkers. Pharmacogenomics, 2014; 15:1611–35. CrossRef
Linda SL, John J, Weixian M, Verica R, Kishor MW, Helen MB. Methotrexate loaded Poly(L-Lactic Acid) microspheres for intra-articular delivery of MTX to the joint. J Pharm Sci, 2004; 93:943–56. CrossRef
Martin M, Bjoern L. Water self-diffusion in aqueous block copolymer solutions. Macromol, 1992; 25:5446–50. CrossRef
Miller SC, Drabik BR. Rheological properties of poloxamer vehicles. Int J Pharm, 1984; 18:269–76. CrossRef
O’Dell JR. Therapeutic strategies for rheumatoid arthritis. N Engl J Med, 2004; 350:2591–602. CrossRef
Oh KT, Bronich TK, Kabanov AV. Micellar formulations for drug delivery based on mixtures of hydrophobic and hydrophilic Pluronic® block copolymers. J Control Rel, 2004; 94(2–3):411–22. CrossRef
Paavola A, Yliruusi J, Rosenberg P. Controlled and durameter permeability of lidocaine and ibuprofen from injectable poloxamer based gels. J Control Rel, 1998; 52:169–78. CrossRef
Rudan I, Sidhu S, Papana A, Meng SJ, Xin-Wei Y, Wang W, Campbell-Page RM, Demaio AR, Nair H, Sridhar D, Theodoratou E. Prevalence of rheumatoid arthritis in low–and middle–income countries: a systematic review and analysis. J Glob Health, 2015; 1:5.
Schmolka IR. Artificial skin: preparation and properties of Pluronic F-127 gels for treatment of burns. J Biomed Mater Res, 1972; 6:571–82. CrossRef
Schwartzman S, Fleischmann R, Morgan JG. Do anti-TNF-∞ agents have equal efficacy in patients with rheumatoid arthritis. Arthr Res Ther, 2004; 6:3–11. CrossRef
Sharma A, Arora S. Formulation and in vitro evaluation of ufasomes for dermal administration of Methotrexate. ISRN pharm, 2012; 2012:1–8. CrossRef
Shinde CG, Venkatesh MP, Kumar TM, Shivakumar HG. Methotrexate: a gold standard for treatment of rheumatoid arthritis. J Pain Palliat Care Pharmacother, 2014; 28:351–8. CrossRef
Singhare DS, Khan S, Yeole PG. Temperature induced in situ gel of lidocaine hydrochloride for periodontial anaesthesia. Indian Drugs, 2005; 42:519–24.
Siriporn T, John G, Frank H, Colin B. Gelation of aqueous solutions of diblock copolymers of ethylene oxide and D, L-lactide. Macromol Chem Physic, 1997; 198:3385–95. CrossRef
Sun S, Cao H, Su H, Tan T. Preparation and characterization of a novel injectable in situ cross-linked hydrogel. Polym Bull, 2009; 62:699–711. CrossRef
Vena GA, Cassano N, Iannone F. Update on subcutaneous methotrexate for inflammatory arthritis and psoriasis. Ther Clin Risk Manag, 2018; 14:105. CrossRef
Venkatesh MP, Anis S, Pramod KTM. Design and development of an injectable in situ forming drug delivery system of methotrexate for the treatment of rheumatoid arthritis. J Drug Deliv Sci Technol, 2013; 23:445–53. CrossRef
Venkatesh MP, Purohit KL, Teggin MP, Shivakumar HG. In situ gels based drug delivery systems. Curr Drug Ther, 2011; 6:213–22. CrossRef
Wigginton S, Chu B, Weisman M, Howell S. Methotrexate pharmacokinetics after intraarticular injection in patients with rheumatoid arthritis. Arthritis Rheum, 1980; 23:119–22. CrossRef
Yannic BS, Robert G, Olivier JA. Novel thermoresponsive hydrogel based on chitosan. Eur J Pharm Biopharm, 2008; 68:19–25. CrossRef
Zaki Rizkalla CM, Latif Aziz, Soliman R II. In-vitro and invivo evaluation of hydroxyzine hydrochloride microsponges for topical delivery. AAPS Pharm Sci Technol, 2011; 12:989–1001. CrossRef