INTRODUCTION
Antibiotic resistance is a worldwide phenomenon. Effective antibiotics are essential for preventing and treating infections [1–3]. Moreover, as a leading cause of morbidity, foodborne infections are among the most severe and expensive public health concerns worldwide. Thus, new antimicrobial compounds are needed to extend food shelf life, reduce food deterioration, and increase food safety [4,5].
Recently, natural products have been developed as an option for synthetic drugs due to their adverse side effects. Numerous plant metabolites have been widely used to cure several diseases [6,7]. Nonetheless, several key medicinal plants are gradually extinct due to overharvesting to meet market demands on new bioactive compounds. Therefore, scientists have begun working with endophytes in recent years as they can produce biomedically essential phytocompounds [8–11].
Mangroves, primarily tropical vegetations that thrive in harsh environmental conditions, support a variety of endophytes [12,13]. They are well known as sources of antibacterial [14,15], anticancer [16], antioxidant [8,17], antifungal [18,19], antiviral [12], enzyme activation and inhibition [20,21], immunosuppressive [22,23], anti-inflammatory [24,25], and antifeedant [12]. Indonesia is home to 20% of mangroves globally (3.2 million hectares), with 45 mangrove species. Furthermore, the province of Riau alone is responsible for approximately 143 billion hectares of this forest [26–28]. Although this archipelago contains the world’s most valuable mangrove ecosystem, only a few studies have been published on exploring Indonesia’s mangrove-derived endophytes for their bioactive compounds of medicinal significance. Nevertheless, no study has been undertaken to explore the potential endophytes derived from mangroves in Riau Province.
Antimicrobial activity from endophytic fungi isolated from mangroves in Indonesia was rarely studied. We have published promising bacterial and fungal endophytes as sources of antimicrobial compounds derived from mangroves in the coastal area of Riau Province [14,29]. In this study, we explored for the first time the diversity of fungal endophytes from mangroves in Siput River Estuaries that exhibit antibacterial activity and their molecular identification through ITS rDNA amplification.
MATERIAL AND METHODS
Sampling site and isolation of culturable endophytes
Eight healthy mangroves with different characters were chosen randomly from the estuaries of Siput River, Bengkalis Regency, Riau Province, Indonesia (1°15?59” LU; 102°8?31” BT). Plant specimens (roots, leaves, twigs, and flowers/fruits) were taken, wrapped in aluminum foil, and sent to University Herbarium for species identification. The root samples for endophytic fungi isolation were brought to the laboratory in separate sterile polyethylene bags in a surface-sterilized box with dried ice. Endophytic fungi were then isolated from each sample’s root immediately after reaching the lab using a standard protocol as described in our previous work [29].
In vitro antagonism test
All isolated endophytes were screened for in vitro antagonism activity against selected pathogenic bacteria to reveal their potential as an antimicrobial compound(s) source. Bacterial indicators used in this analysis including Gram-positive (Staphylococcus aureus ATCC29213 and Bacillus subtillis ATCC11774) and Gram-negative (Escherichia coli ATCC35218, Vibrio parahaemolyticus, and Vibrio alginolyticus), the culture collection of the Biochemistry and Molecular Biology Laboratory, University of Riau. A plug of 5-day-old culture (6 mm diameter) of each fungal endophyte was inoculated on the surface of Mueller–Hinton Agar (MHA; Oxoid, Hampshire, England) containing each pathogenic bacteria (107 CFU/plate) and incubated overnight at 37°C. MHA plates containing each pathogen without fungal endophyte were used as the negative control. All procedures were carried out in triplicate. The diameter of the inhibition zone (in mm) was measured using a caliper after 24 hours of incubation. Endophytes showing broad-spectrum antibacterial activity with high inhibition zone were then identified based on morphology on potato dextrose agar (PDA; Oxoid, Hampshire, England) plate and their ribosomal RNA gene’s internal transcribed spacer (ITS) regions using universal primers (IDT, Coralville, IA).
ITS region amplification and sequencing
Each isolated endophyte was cultured on potato dextrose agar. Genomic DNA from each endophyte was isolated using Geneaid GBYB100 Presto Mini gDNA Yeast Kit. Amplification of ITS region was performed using 10 μM universal primers ITS4 (ITS4-R 5?- TCC TCC GCT TAT TGA TAT GC-3?) and ITS5 (ITS5-F 5?- GAA AGT AAA AGT CGT AAC AAG G-3?) at 50.5°C annealing temperature for 45 seconds. Negative control (absence of DNA template) was used to detect DNA contamination.
Sequencing was carried out at Genetica Science Indonesia. The obtained sequences were compared to those available in GenBank via the Basic Local Alignment Search Tool (BLAST) for final identification. Multialignment and construction of sequence differences for edited sequences obtained in this study and sequences from the GeneBank database at the National Center for Biotechnology Information Nucleotide Sequence database were performed using BioEdit software. The phylogenetic tree was built using the neighbor-joining algorithm and bootstrapping of 1,000 replications by MEGA6.
Phytochemical analysis
For this analysis, small-scale fermentation of Botryosphaeria rhodina was first carried out, followed by extraction using ethyl acetate. Fermentation in solid rice medium and secondary metabolites extraction was performed according to the procedure described by Kjer et al. [30]. Solid rice medium was prepared by adding 200 g rice with 210 ml water in a 2,000 ml Erlenmeyer flask and sterilized at 121°C for 15 minutes. A plug with a size of ~1.5 × 1.5 cm was taken from a pure fungal strain that was freshly grown and covered the surface of the inoculated PDA plate and transferred into an Erlenmeyer flask containing sterile solid rice medium. The culture was incubated at room temperature for 10 days.
The culture medium containing the mycelium was then cut into small pieces, extracted with ethyl acetate, and filtered under vacuum using a Buchner funnel. The ethyl acetate extract was then subjected to phytochemical analysis to determine the presence of alkaloids, terpenoids, steroids, saponins, phenols, and flavonoids in accordance with Harborne [31] and Akhtar et al. [32].
Statistical analysis
Antibacterial activity was expressed as inhibition zones formed around the endophytes and presented as mean ± standard deviation of three replicates. One-way analysis of variance was run using Minitab® software version 19 (Pennsylvania, USA) to determine the significant differences, followed by Tukey’s pairwise comparison at α = 5%.
RESULTS
Isolation of culturable endophytes from the roots of mangroves
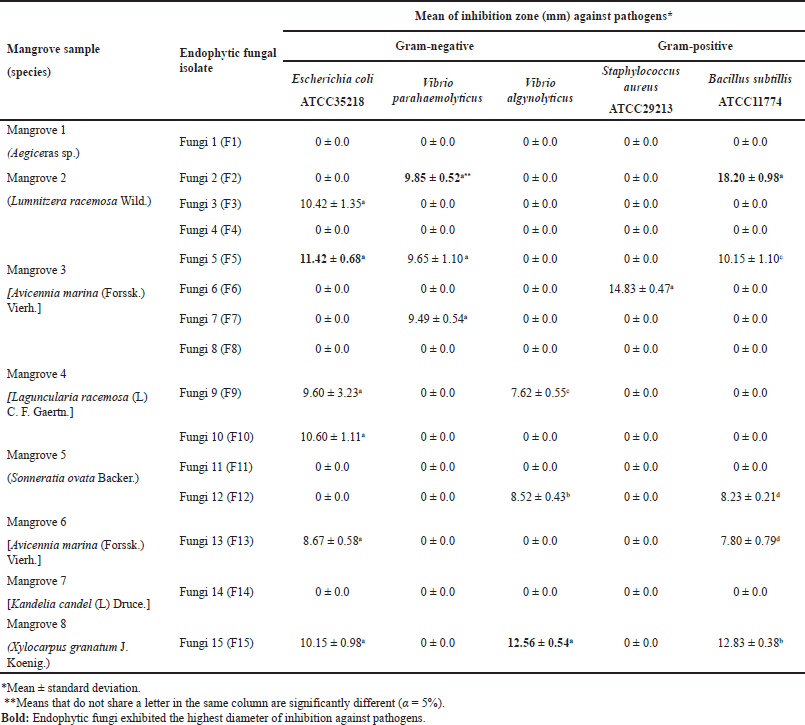
Figure 1 shows the sampling location of Siput River estuaries in Siak Kecil Regency, Bengkalis. As shown in Table 1, a total of 15 fungal endophytes were isolated from the roots of eight mangrove samples, which were identified as Aegiceras sp. (1 isolate), Lumnitzera racemosa Wild. (2 isolates), Avicennia marina (Forssk.) Vierh. (6 isolates), Laguncularia racemosa (L) C. F. Gaertn. (1 isolate), Sonneratia ovata Backer. (3 isolates), Kandelia candel (L) Druce. (1 isolate), and dan Xylocarpus granatum J. Koenig (1 isolate).
Antagonistic activity of endophytes against Gram-positive and Gram-negative bacteria
Table 1 depicts the result of in vitro antagonism activity of 15 isolated endophytes. The mean of inhibition zones was compared among endophytes against each pathogen. Out of 15 isolates tested, ten isolates actively inhibited the growth of at least one pathogen, and E. coli was found to be the most inhibited. The highest inhibition against V. alginolyticus was shown by F15, while F2 was found to be the most potential isolate to produce a compound to inhibit the growth of B. subtilis. Isolates F15, F2, and F5 were the most potential producers for compounds with antimicrobial activity as they exhibited high inhibition against both Gram-positive and Gram-negative bacteria.
Figure 2 presents the macroscopic of those isolates in potato dextrose agar (upper side of each isolate), and their microscopic character (lower side). F2 isolate grew as a floccose-white colony with branched and septate mycelium, while F5 colony was gray-green with conidiophores. The F15 plate showed greyish-white color mycelium that grew fast. They need 3–4 days to cover the surface of the agar in 90 mm Petri plates. At later growth stages, they turned black due to enormous spore production.
ITS region amplification and sequencing
Our results showed that PCR analysis using ITS1 and ITS4 primers generated a single band of ~550–600 bp. The original neighbor-joining tree with 1,000 bootstrap replications is shown in Figure 3. Based on sequence similarity as determined by the BLAST program in the GenBank database, and according to the phylogeny tree, the closest species of isolates F2, F5, and F15 is Fusarium equiseti isolate FUS-34-2 (Accession no. MH879250.1; 100%), Aspergillus fumigatus strain DTO 402-H1 (Accession no. MT316338.1; 100%), and B. rhodina isolate P130 (Accession no. EF423547.1; 99.82%), respectively.
Phytochemical analysis
Table 2 shows the phytochemical analysis data of the secondary metabolites in the B. rhodina extract. Our finding confirmed the presence of terpenoid, phenolic, and flavonoid.
DISCUSSION
Endophytes are microorganisms that live inside plant tissues without harming the host plant. These microorganisms can be bacteria, fungi, or viruses and can exist in various plant tissues, including leaves, stems, and roots. Endophytes have been demonstrated to contribute to plant growth, health, and resilience to various biotic and abiotic stresses. Endophytes and their correlation with antimicrobial activity in numerous plant species, including mangroves, have been extensively researched [33,34]. Mangroves are a group of salt-tolerant trees that grow in the intertidal zones of tropical and subtropical coasts. Mangroves have diverse environmental impacts, including protecting soils from floods and cyclones, preserving the integrity of river banks, and promoting biodiversity. These ecosystems also yield various natural products with potential benefits and commercial value. Furthermore, mangroves have a history of providing medicinal and food products. The natural products sourced from mangroves have various applications in areas such as agriculture, aquaculture, reforestation, and infrastructure [35].
Mangroves have been proven to harbor various endophytes, many of which produce antimicrobial compounds. The unique environmental conditions found in mangroves, such as high salinity, temperature fluctuations, and limited nutrient availability, may have led to the evolution of unique endophyte communities with specialized metabolic capabilities, including the production of antimicrobial compounds [36,37].
 | Figure 1. Elevation map for siput River, Siak Kecil, Bengkalis Regency, Riau Province, Indonesia. [Click here to view] |
 | Table 1. Mangrove samples and isolated fungal endophytes’ antimicrobial activities. [Click here to view] |
Riau Province is located in the central part of Sumatra Island, Indonesia. This province covers 8,702,366 hectares of total area, with Pekanbaru as the capital city. As reported by the Forestry Ministry service of Riau, this province has 138,434 hectares of mangrove forest, constituting 21.6% of Indonesia’s total mangrove area. Bengkalis is one of 10 regencies in Riau Province with 21,981 hectares of mangrove forest. However, no scientific paper reports the exploration of endophytes from Riau Mangrove as a potential source of antimicrobial compounds. For the first time, this work discusses the endophytes of mangroves from Siput River estuaries in Siak Kecil Regency, Bengkalis, Indonesia. This area is located around the border of the Malacca Strait (Fig. 1).
The results of the in vitro antagonism test showed that ten isolates were able to inhibit the growth of at least one pathogen. Isolate F15 was the best isolate based on the One-Way Analysis of Variance (α = 5%) in comparing the mean of clear zone inhibition diameter toward all pathogens tested, followed by F2 and F5. Figure 2 shows the macroscopic of those isolates in potato dextrose agar and their microscopic character. The macroscopic and microscopic image depicted that the pure culture of isolate F2 was a floccose-white colony with smooth, branched, cylindrical, and septate mycelium. The morphology of isolate F5 showed a spore-forming mold fungus with grey-green colored colonies, conidiophores, and subglobose conidia. In comparison, it was shown that the mycelium of isolate F15 grew vigorously on the surface of PDA. In 3–4 days, the surface of the PDA has been fully covered by its greyish-white aerial mycelia. These data indicated the identity of genus Fusarium, Aspergillus, and Botryospharia.
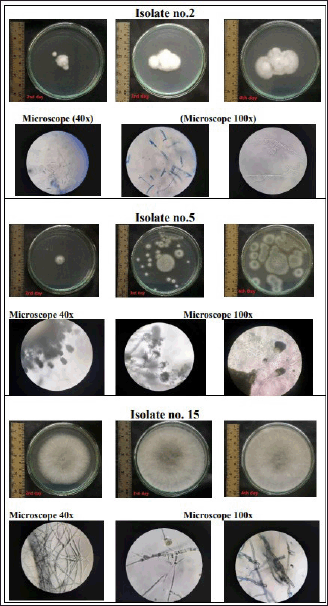 | Figure 2. The macroscopic and microscopic character of F2, F5, and F15 isolates. The macroscopic phenotype of a microorganism refers to its observable physical characteristics, such as its shape, size, and colony formation, while the microscopic phenotype is its internal cellular and molecular structures that can only be observed through the use of microscopes. [Click here to view] |
 | Figure 3. Maximum likelihood phylogenetic tree with 1000 bootstrap-replications. [Click here to view] |
Endophytes enhance the physiology of their host plants, which results in improved health compared to plants without them [38]. This is because endophytes release substances such as cytokines, phytohormones, and other plant growth-promoting compounds, which boost the growth of host plants’ growth directly or indirectly. Moreover, endophytes can produce compounds that were formerly considered synthesized by plants. This is probably due to the horizontal gene transfer from plant to endophyte genome or vice versa [39]. Numerous endophytes can produce a variety of bioactive metabolites that can be used as therapeutic agents against various diseases, either directly or indirectly. Furthermore, diverse microorganisms in plant tissues enable microbial interactions such as quorum sensing, resulting in the production of metabolites [40].
Mangrove-derived endophytes are rich sources of various pharmacologically active metabolites, as mangroves produce chemically unique secondary metabolites that are diverse in nature [16,35]. The presence of alkaloids has been reported from the mangrove endophytic fungus Phomopsis sp. SYSUQYP-23 and Didymella sp. CYSK-4 [25,41]. Other researchers found that endophytes from mangroves produced coumarins, terpenoids [42], and polyketides [43,44].
Advances in molecular genetics allowed the incorporation of DNA analysis into the taxonomy. In this study, a molecular approach for species differentiation was performed to amplify the ITS region, a powerful tool to characterize fungal diversity that is located between the highly conserved genes coding for 18S and 28S rRNA [45,46]. The regions are characterized by a high degree of fungi heterogeneity and have been reported to be useful in mold phylogenetic and taxonomic analyses [47,48]. Our finding showed amplicons with a size of ~550–600 bp. The 16S rRNA sequence-based phylogenetic tree analysis (Fig. 3) revealed that the lineages of the isolates could be divided into three main groups, representing different genera, i.e., Botryosphaeria, Fusarium, and Aspergillus. Nucleotide BLAST results showed that the F2, F5, and F15 are Fusarium equiseti, A. fumigatus, and B. rhodina, respectively.
All species found in this study were previously reported as endophytic fungi. The genus Fusarium is widely distributed throughout the world in the form of pathogenic and nonpathogenic strains. Fusarium equiseti is an endophyte fungus that has been found in rice [49], medicinal plant Cananga odorata, barley roots [50], and Limonium cossonianum from a coastal salt marsh [51]. The presence of the genus Fusarium in mangroves has been reported from Kandelia candel and Podophyllum hexandrum [35]. The genus Fusarium is a potential fungi producer of novel antibiotics [50]. Our finding showed that isolate F2, Fusarium equiseti from mangrove L. racemosa Wild, had a bigger clear zone toward B. subtilis ATCC11774 than E. coli ATCC35218. This result is in agreement with other researchers who reported that Fusarium equiseti has better inhibition against gram-positive bacteria due to the presence of an antibiotic like “enniatins.”
 | Table 2. Phytochemical analysis data of B. rhodina extract. [Click here to view] |
Aspergillus species have been defined as endophytes, saprophytes, parasites, and human pathogens, all of which exhibited a high propensity for producing secondary metabolites with diverse chemical structures and bioactivities [52]. In a previous report, endophytic A. fumigatus was isolated from mangrove Sonneratia griffithii Kurz in West Sumatra, Indonesia [15], and in inland salt marshes [51]. In the present study, A. fumigatus was isolated from the mangrove Avicennia marina (Forssk.) Vierh. and denoted the potential of broad-spectrum activity as it suppressed the growth of E. coli ATCC35218, V. parahaemolyticus, and B. subtilis ATCC11774. Our finding is in line with previously published papers that reported the wide-range activity of metabolites from A. fumigatus endophyte, for instance, heterocyclic alkaloids as antimicrobial agents [53,52], immunosuppressive agents [22]. Much attention has been paid to a particular group of endophytic fungi belonging to Botryosphaeria in recent years. Our study found that the most potential isolate, isolate F15, is B. rhodina from mangrove Xylocarpus granatum J. Koenig. Botryosphaeria rhodina has been reported previously in Mangrove Kandelia candel [54] and the leaves of Garcinia mangostana [55]. Other researchers isolated the group of Botryosphaeria sp. in the medicinal plant Bidens pilosa [56].
Metabolites from Botryosphaeria species are some notable classes of chemical moieties, including naphthalenones, lactones, polyketides, diterpenoids, benzofuran derivatives, and exopolysaccharides [55] that have potential antibacterial properties [57]. Antibacterial activity of B. rhodina is probably due to the presence of antibacterial primin, as well as several novel compounds such as alkaloids, coumarins, ceramides, lactones, diterpenoids, benzofuran derivatives, meroterpenoids, polyketides, and polysaccharides [54].
The results of the antagonistic test against multiple pathogenic bacteria showed that B. rhodina possesses a high degree of inhibiting ability. Therefore, a small-scale fermentation followed by ethyl acetate extraction was performed. As shown in Table 2, this extract was subjected to identification testing for secondary metabolite compounds. These tests revealed that B. rhodina can synthesize secondary metabolite compounds belonging to the terpenoid, phenolic, and flavonoid classes. Botryoisocoumarin A, a phenolic compound isolated from B. rhodina, demonstrated antibacterial activity, as reported by Ju et al. [58]. Botryorhodines A–D, which are also phenolic compounds, have antimicrobial activity, according to Abdou et al. [56]. Based on the results of this phytochemical analysis, a preliminary conclusion can be drawn that the antibacterial activity of B. rhodina is derived from secondary metabolites such as phenolic compounds, and this preliminary result can serve as a guide for future research on isolating secondary metabolites from this species.
CONCLUSION
Endophytes have been widely recognized as important components of the mangrove ecosystem and have been shown to play a crucial role in plant growth, health, and disease resistance. Isolation of endophytes from mangroves has revealed a diverse community of microorganisms that produce a variety of antimicrobial compounds with activity against a range of pathogens. This study has identified three endophytic fungi from Mangrove L. racemosa Wild., Avicennia marina (Forssk.) Vierh., and Xylocarpus granatum J. Koenig, which are potential sources of wide spectrum antibacterial agents. These fungi have close relatedness with Fusarium equiseti isolate FUS-34-2 (100%), A. fumigatus strain DTO 402-H1 (100%), and B. rhodina isolate P130 (99.82%), respectively. The ethyl acetate extract of B. rhodina, the most potential isolate, was found positive for the presence of terpenoid, phenolic, and flavonoid. This report highlights the importance of ongoing efforts to isolate, study, and understand the diversity and functions of endophytes in mangroves and other plant species.
Declaration of Competing Interest
The authors declare that no competing personal and/or financial interests influenced the works reported in this paper.
ACKNOWLEDGMENT
The authors thank the Indonesian Ministry of Higher Education through the Riau University Research and Community Development Centre, and Riau University for their financial supports.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
This work was supported by a Fundamental Research Grant from the Indonesian Ministry of Higher Education through the Riau University Research and Community Development Centre (Grant no. 063/SP2H/LT/DRPM/2020). APC was supported by DIPA PNBP FMIPA Universitas Riau 2023.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Morehead MS, Scarbrough C. Emergence of global antibiotic resistance. Primary Care. 2018;45(3):467–84. https://doi.org/10.1016/j.pop.2018.05.006
2. Ragheb MN, Thomason MK, Hsu C, Nugent P, Gage J, Samadpour AN, et al. Inhibiting the evolution of antibiotic resistance. Mol Cell. 2019;73(1):157–65. doi: https://doi.org/10.1016/j.molcel.2018.10.015
3. Yadav S, Kapley A. Antibiotic resistance: global health crisis and metagenomics. Biotechnol Rep. 2021;29:e00604. doi: https://doi.org/10.1016/j.btre.2021.e00604
4. Eghbal N, Viton C, Gharsallaoui A. Food bioscience nano and microencapsulation of bacteriocins for food applications?: a review. Food Biosc. 2022;50(102173):1–15. doi: https://doi.org/10.1016/j.fbio.2022.102173
5. O’Connor PM, Kuniyoshi TM, Oliveira RP, Hill C, Ross RP, Cotter PD. Antimicrobials for food and feed; a bacteriocin perspective. Curr Opin Biotechnol. 2020;61:160–7. doi: https://doi.org/10.1016/j.copbio.2019.12.023
6. Aziz MM, Raza MA, Saleem H, Wajid M, Bashir K, Ikram M. Medicinal values of herbs andpplants, importance of phytochemical evaluation and ethnopharmacological screening: an illustrated review essay. J Pharm Cosmet Sci. 2014;2(1):6–10.
7. Newman DJ, Cragg GM. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J Nat Product. 2020;83(3):770–803. doi: https://doi.org/10.1021/acs.jnatprod.9b01285
8. Bibi SN, Gokhan Z, Rajesh J, Mahomoodally MF. Fungal endophytes associated with mangroves—chemistry and biopharmaceutical potential. South Afr J Botany. 2020;134:187–212. doi: https://doi.org/10.1016/j.sajb.2019.12.016
9. Gouda S, Das G, Sen SK, Shin HS, Patra JK. Endophytes?: a treasure house of bioactive compounds of medicinal importance. Front Microbiol.2016; 7:1–8. https://doi.org/10.3389/fmicb.2016.01538
10. Ludwig-Muller J. Plants and endophytes: equal partners in secondary metabolite production?? Biotechnol Lett. 2015;37(7):1325–34. doi: https://doi.org/10.1007/s10529-015-1814-4
11. Porras-Alfaro A, Bayman P. Hidden fungi, emergent properties: endophytes and microbiomes. Annu Rev Phytopathol. 2011;49:291–315. doi: https://doi.org/10.1146/annurev-phyto-080508-081831
12. Deshmukh SK, Prakash V, Ranjan N. Marine fungi: a source of potential anticancer compounds. Front Microbiol. 2018;8(2536):1–24. doi: https://doi.org/10.3389/fmicb.2017.02536
13. Singh YD, Singh MC, Panda MK. Biotechnological aspects of mangrove microorganisms. Biotechnol Utilization Mangrove Res. 2020;381–398. doi: https://doi.org/10.1016/b978-0-12-819532-1.00018-4
14. Handayani D, Rivai H, Hutabarat M, Rasyid R. Antibacterial activity of endophytic fungi isolated from mangrove plant Sonneratia griffithii Kurz. J Appl Pharma Sci. 2017;7(4):209–12. doi: https://doi.org/10.7324/JAPS.2017.70431
15. Haryani Y, Hilma R, Delfira N, Martalinda T, Puspita F, Friska A, et al. Anti-vibriosis activity of endophytic fungi associated with Ceriops tagal (Perr.) C.B.Rob and Bruguiera sp., mangrove plants from Riau Province, Indonesia. AIP Conf Proc. 2020;1:2243. https://doi.org/10.1063/5.0001446
16. Das SK, Samantray D, Thatoi HN. Pharmacological applications of metabolites of mangrove endophytes: a review. Microb Biotechnol. 2018;2:331–60. doi: https://doi.org/10.1007/978-981-10-7140-9_16
17. Narendran R, Kathiresan K. Antimicrobial activity of crude extracts from mangrove-derived Trichoderma species against human and fish. Biocatal Agric Biotechnol. 2016;6:189–94. doi: https://doi.org/10.1016/j.bcab.2016.03.003
18. Costa IPMW, Maia LC, Cavalcanti MA. Diversity of leaf endophytic fungi in mangrove plants of Northeast Brazil. Brazil J Microbiol. 2012;43(3):1165–73. doi: https://doi.org/10.1590/S1517-83822012000300044
19. Hu Z, Wu Z, Su Q, Li M, Wu S, Meng R, et al. Metabolites with phytopathogenic fungi inhibitory activities from the mangrove endophytic fungus Botryosphaeria ramose. Bioorg Chem. 2020;104:104300. doi: https://doi.org/10.1016/j.bioorg.2020.104300
20. Liu Y, Yang Q, Xia G, Huang H, Li H, Ma L, et al. Polyketides with α-glucosidase inhibitory activity from a mangrove endophytic fungus, Penicillium sp. HN29-3B1. J Nat Prod. 2015;78(8):1816–22. doi: https://doi.org/10.1021/np500885f
21. Maria GL, Sridhar KR, Raviraja NS. Antimicrobial and enzyme activity of mangrove endophytic fungi of southwest coast of India. J Agric Technol. 2005;1(1):67–80.
22. Xu ZY, Zhang XX, Ma JK, Yang Y, Zhou J, Xu J. Secondary metabolites produced by mangrove endophytic fungus Aspergillus fumigatus HQD24 with immunosuppressive activity. Biochem Systemat Ecol. 2020;93:104166. doi: https://doi.org/10.1016/j.bse.2020.104166
23. Liu H, Chen S, Liu W, Liu Y, Huang X, She Z. Polyketides with immunosuppressive activities from mangrove endophytic fungus Penicillium sp. ZJ-SY2. Mar Drugs. 2016;14(12):1–7. doi: https://doi.org/10.3390/md14120217
24. Chen S, Ding M, Liu W, Huang X, Liu Z, Lu Y, et al. Anti-inflammatory meroterpenoids from the mangrove endophytic fungus Talaromyces amestolkiae YX1. Phytochemistry. 2018;146:8–15. doi: https://doi.org/10.1016/j.phytochem.2017.11.011
25. Chen Y, Yang W, Zou G, Yan Z, Qiu P, Long Y, et al. Metabolites with anti-inflammatory and α-glucosidase inhibitory activities from the mangrove endophytic fungus Phoma sp. SYSU-SK-7. Tetrahedron Lett. 2020;61(48):152578. doi: https://doi.org/10.1016/j.tetlet.2020.152578
26. Giri C, Ochieng E, Tieszen LL, Zhu Z, Singh A, Loveland T, et al. Status and distribution of mangrove forests of the world using earth observation satellite data. Glob Ecol Biogeogr. 2011;20(1):154–9. doi: https://doi.org/10.1111/j.1466-8238.2010.00584.x
27. Ilman M, Dargusch P, Dart P, Onrizal. A historical analysis of the drivers of loss and degradation of Indonesia’s mangroves. Land Use Policy. 2016;54:448–59. doi: https://doi.org/10.1016/j.landusepol.2016.03.010
28. Nordhaus I, Toben M, Fauziyah A. Impact of deforestation on mangrove tree diversity, biomass and community dynamics in the Segara Anakan Lagoon, Java, Indonesia: a ten-year perspective. Estuar. Coast. Shelf Sci. 2019;227:106300. doi: https://doi.org/10.1016/j.ecss.2019.106300
29. Haryani Y, Hilma R, Delfira N, Martalinda T, Puspita F, Friska A, et al. Antibacterial activity of Achromobacter sp. and Bacillus sp., bacterial endophytes derived from mangrove Ceriops tagal (Perr.) C.B.Robb. IOP Conf Ser: Mater Sci Eng. 2020;833(1):1–6. doi: https://doi.org/10.1088/1757-899X/833/1/012013
30. Kjer J, Debbab A, Aly AH, Proksch P. Methods for isolation of marine-derived endophytic fungi and their bioactive secondary products. Nat Protocols. 2010;53:479–90.
31. Harborne, A. Phytochemical methods a guide to modern techniques of plant analysis. Berlin, Germany: Springer Science & Business Media; 1998.
32. Akhtar N, Mirza B. Phytochemical analysis and comprehensive evaluation of antimicrobial and antioxidant properties of 61 medicinal plant species. Arabian J Chem. 2018;11(8):1223–35.
33. Kandasamy GD, Kathirvel P. Insights into bacterial endophytic diversity and isolation with a focus on their potential applications—a review. Microbiol Res. 2022;266:127256.
34. Maia LKR, Alves DR, Junior SGJ, de Morais SM, de Oliveira Freire FDC, do Nascimento Bordallo P, et al. Identification and characterization of endophytic fungi found in plants from northeast Brazilian mangroves: a review. Res Soc Dev. 2022;11(7):e5111729459.
35. Chatterjee A, Abraham J. Mangrove endophytes: a rich source of bioactive substances. Biotechnol Utilization Mangrove Res. 2020;27–47. doi: https://doi.org/10.1016/b978-0-12-819532-1.00002-0
36. Chen S, Cai R, Liu Z, Cui H, She Z. Secondary metabolites from mangrove-associated fungi: source, chemistry and bioactivities. Nat Prod Rep. 2022;39(3):560–95.
37. Narayanan MM, Shivanand P, Ahmad N. Pharmacological maneuver of mangrove endophytic fungi in the South China sea—a review. J Trop Biodivers Biotechnol. 2022;7(2):jtbb69913.
38. Waller F, Achatz B, Baltruschat H, Fodor J, Becker K, Fischer M, et al. The endophytic fungus Piriformospora indica reprograms barley to salt-stress tolerance, disease resistance, and higher yield. Proc Natl Acad of Sci. 2005;102(38):13386–91.
39. Alam B, L? J, G? Q, Khan MA, G?ng J, Mehmood S, et al. Endophytic fungi: from symbiosis to secondary metabolite communications or vice versa? Front Plant Sci. 2021;12:3060.
40. Kusari P, Kusari S, Spiteller M, Kayser O. Implications of endophyte-plant crosstalk in light of quorum responses for plant biotechnology. Appl Microbiol biotechnol. 2015;99:5383–90. doi: https://doi.org/10.1007/s00253-015-6660-8
41. Chen Y, Liu Z, Huang Y, Liu L, He J, Wang L, et al. Ascomylactams A-C, cytotoxic 12- or 13-membered-ring macrocyclic alkaloids isolated from the mangrove endophytic fungus Didymella sp. CYSK-4, and structure revisions of phomapyrrolidones A and C. J Nat Prod. 2019;82(7):1752–8. doi: https://doi.org/10.1021/acs.jnatprod.8b00918
42. Deng Q, Li G, Sun M, Yang X, Xu J. A new antimicrobial sesquiterpene isolated from endophytic fungus Cytospora sp. from the Chinese mangrove plant Ceriops tagal endophytic fungus Cytospora sp. from the Chinese. Nat Prod Res. 2018;34(10):1–5. doi: https://doi.org/10.1080/14786419.2018.1512993
43. Yan Z, Wen S, Ding M, Guo H, Huang C, Zhu X, et al. The purification, characterization, and biological activity of new polyketides from mangrove-derived endophytic fungus Epicoccum nigrum SCNU-F0002. Mar Drugs. 2019;17(7):414.
44. Yu X, Müller WEG, Meier D, Kalscheuer R, Guo Z, Zou K, et al. Polyketide derivatives from mangrove derived endophytic fungus Pseudopestalotiopsis theae. Mar Drugs. 2020;18(2):1–15. doi: https://doi.org/10.3390/md18020129
45. Abarenkov K, Somervuo P, Nilsson RH, Kirk PM, Huotari T, Abrego N, et al. Protax-fungi: a web-based tool for probabilistic taxonomic placement of fungal internal transcribed spacer sequences. New Phytol. 2018;220(2):517–25. doi: https://doi.org/10.1111/nph.15301
46. Schoch CL, Seifert KA, Huhndorf S, Robert V, Spouge JL, Levesque CA, et al. Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for fungi. Proc Natl Acad Sci USA. 2012;109(16):6241–6. doi: https://doi.org/10.1073/pnas.1117018109
47. Ciardo DE, Schär G, Böttger EC, Altwegg M, Bosshard PP. Internal transcribed spacer sequencing versus biochemical profiling for identification of medically important yeasts. J Clin Microbiol. 2006;44(1):77–84. doi: https://doi.org/10.1128/JCM.44.1.77-84.2006
48. Trabelsi H, Neji S, Hadrich I, Khemakhem N, Sellami H, Makni F, et al. Contribution of the internal transcribed spacer regions to the detection and identification of human fungal pathogens. Curr Res Transl Med. 2019;67(3):100–6. doi: https://doi.org/10.1016/j.retram.2019.04.001
49. Mane RS, Paarakh PM, Vedamurthy AB. Brief review on fungal endophytes. Int J Second Metab. 2018;5(4):288–303. doi: https://doi.org/10.21448/ijsm.482798
50. Macia-Vicente JG, Rosso LC, Ciancio A, Jansson HB, Lopez-Llorca LV. Colonisation of barley roots by endophytic Fusarium equiseti and Pochonia chlamydosporia: effects on plant growth and disease. Ann Appl Biol. 2009;155(3):391–401. doi: https://doi.org/10.1111/j.1744-7348.2009.00352.x
51. Maciá-Vicente JG, Jansson HB, Abdullah SK, Descals E, Salinas J, Lopez-Llorca LV. Fungal root endophytes from natural vegetation in Mediterranean environments with special reference to Fusarium spp. FEMS Microbiol Ecol. 2008;64(1):90–105. doi: https://doi.org/10.1111/j.1574-6941.2007.00443.x
52. Zhang H, Ruan C, Bai X, Chen J, Wang H. Heterocyclic Alkaloids as antimicrobial agents of Aspergillus fumigatus endophytic on Edgeworthia chrysantha. Chem Nat Compound. 2018;54(2): 411–4. doi: https://doi.org/10.1007/s10600-018-2365-4
53. Flewelling AJ, Bishop AL, Johnson JA, Gray CA. Polyketides from an endophytic Aspergillus fumigatus isolate inhibit the growth of Mycobacterium tuberculosis and MRSA. Nat Product Commun.2015; 10(10):1661–2. doi: https://doi.org/10.1177/1934578x1501001009
54. Ju ZR, Qin X, Lin XP, Wang JF, Kaliyaperumal K, Tian YQ, et al. New phenyl derivatives from endophytic fungus Botryosphaeria sp. SCSIO KcF6 derived of mangrove plant Kandelia candel. Nat Prod Res. 2016;30(2):192–8. doi: https://doi.org/10.1080/14786419.2015.1050670
55. Rukachaisirikul V, Arunpanichlert J, Sukpondma Y, Phongpaichit S, Sakayaroj J. Metabolites from the endophytic fungi Botryosphaeria rhodina PSU-M35 and PSU-M114. Tetrahedron. 2009;65(51):10590–95. doi: https://doi.org/10.1016/j.tet.2009.10.084
56. Abdou R, Scherlach K, Dahse HM, Sattler I, Hertweck C. Botryorhodines a–D, antifungal and cytotoxic depsidones from Botryosphaeria rhodina, an endophyte of the medicinal plant bidens pilosa. Phytochemistry. 2010;711:110–6.
57. Pongcharoen W, Rukachaisirikul V, Phongpaichit S, Sakayaroj J. A new dihydrobenzofuran derivative from the endophytic fungus Botryosphaeria mamane PSU-M76. Chem Pharm Bull. 2017;55(9):1404–5.
58. Ju Z, Lin X, Lu X, Tu Z, Wang J, Kaliyaperumal K, et al. Botryoisocoumarin a, a new Cox-2 Inhibitor from the mangrove Kandelia candel endophytic fungus Botryosphaeria sp. Kcf6. J Antibiot. 2015;6810:653–56.