INTRODUCTION
Multi-drug resistant microbial infection, a major problem in the treatment of infectious disease, is increasing considerably from the last decades (Ahmad et al., 2012; Makki et al., 2015) and to overcome this problem, development of new antimicrobial agents is the need of the hour (Lindahl, 2015; Venkatesh et al., 2016). Hydrazones, belonging to Schiff base family with chemical formula R2C=NNR2, are of incredible interest because of their various biological activities reported in literature, viz., anti-bacterial, anti-fungal, anti-convulsant, anti-inflammatory, anti-cancer, anti-viral, anti-malarial & anti-tuberculosis, anti-oxidant, and antidepressant (Alam et al., 2012; Kumar and Chauhan, 2014; Marwa and Ahmed, 2018; Neda et al., 2018; Nurkenov et al., 2017; Popiolek, 2017; Rayam et al., 2015; Verma et al., 2014). Along with the diverse activities, their ease of synthesis, increased stability, and tendency toward crystallinity make hydrazones a perfect choice to synthesize more effective and novel antimicrobial agents (Bala et al., 2011; 2013; Singh et al., 2016).
The antimicrobial (Chaudhary et al., 2008; Kapoor and Dahiya, 2016; Khatkar et al., 2017) and anti-oxidant (Velika and Kron, 2012; Pontiki and Litina, 2019) perspective of organic acids can be well recognized from the literature. Phenolic compounds (Abouzeed et al., 2018; Benslama et al., 2017; Fu et al., 2016; Sahloul et al., 2014) are also proved as successful antibacterial as well as anti-oxidant activity which might be due to the fact that anti-oxidants act by creating scavenging environment which may inhibit bacterial growth. p-hydroxy benzoic acid, a phenolic derivative, is also found to possess various pharmacological activities, viz., antimicrobial, antialgal, antisickling, antimutagenic, antiviral, anti-inflammatory, anti-oxidant, etc., (Manuja et al., 2013). Antimicrobial potential of p-hydroxy benzoic acid derivatives, viz., Nʹ-(4-methoxybenzylidene)-4-hydroxybenzohydrazide, Nʹ-benzylidene-4-hydroxybenzohydrazide have also been reported (Sapra et al., 2014; Suzana et al., 2017). Therefore, the present study was designed with the aim of synthesizing novel hydrazone derivatives of p-hydroxy benzoic acid for exploring their antimicrobial and anti-oxidant potential.
MATERIALS AND METHODS
Ester of 4-hydroxy benzoic acid was synthesized using Fischer esterification which was further treated with hydrazine hydrate in ethanol to yield corresponding 4-hydroxybenzoic acid hydrazides. The synthesized hydrazides were further reacted with substituted aldehydes (aromatic) in presence of glacial acetic acid (2–3 drops) to yield 4-hydroxy-Nʹ-[(1E)-substituted phenylmethylidene] benzohydrazide (AR1–AR10) (Scheme 1) which were then characterized by spectral and analytical means (Harer et al., 2010; Narang et al., 2012; Rajput and Rajput, 2009).
Evaluation of antibacterial activity
Antibacterial activity of synthesized products was evaluated against Escherichia coli (ATCC 25922), Pseudomonas aeruginosa (ATCC 27853), Staphylococcus aureus (ATCC 25923), and Enterococcus faecalis (ATCC 29212) using Norfloxacin standard as it is the most widely used antibiotic in bacterial infections (Table 2) (Cappucino and Sherman, 1999) by tube dilution method using double strength nutrient broth IP followed by incubation at 37°C ± 1°C.
Antioxidant activity
Hydrogen peroxide radical scavenging (H2O2) assay
Hydrogen peroxide plays an important role as bactericidal agent (Halliwell, 1995) by acting directly or indirectly via its reduction product, OH- (second messenger in synthesis and activation of several inflammatory mediators) (Sprong et al., 1998).
Anti-oxidant activity by the H2O2 method was determined as per the method reported (Ruch et al., 1989). Different dilutions of synthesized (20–60 μg/ml), as well as standard compound (Ascorbic acid) in distilled water, were added to 40 mM H2O2 solution prepared in phosphate buffer and absorbance was measured at 230 nm after 10 minutes against a blank solution.
DPPH radical scavenging activity
DPPH radical scavenging, a standard rapid assay for antioxidant studies (Soares et al., 1977), was done by adding ethanolic DPPH solution (0.1 Mm) to test solutions as well as standard compound to make different dilutions (20–60 μg/ml) and then by measuring their absorbance at 517 nm after 30 minutes. Scavenging activity in both methods was expressed as inhibition percentage and calculated using the following formula:
% inhibition = (Ao−At)/ Ao × 100
where Ao is the absorbance control (blank); At is the absorbance of the test (Shukla et al., 2009).
.png) | Scheme 1. Synthesis of 4-hydroxy-N'-[(1E)-substituted-phenylmethylidene] benzohydrazide analogs. [Click here to view] |
RESULTS AND DISCUSSION
Melting points of synthesized compounds were determined (Elico melting point apparatus, India) and purity was ascertained using single spot TLC (Table 1). The structures were confirmed by spectral studies (IR spectra on FTIR-Shimadzu spectrometer and 1H NMR spectra on BRUKER AVANCE II 400 NMR spectrometer using DMSO). The data obtained from spectral studies were in agreement with assigned molecular structures.
Analytical data for compound AR1
M.P.(°C): 243–247; Yield: 77.4%; IR (cm−1): 1,738.90 (C=O stretch), 1,627.99 (C=N stretch), 3,731.45 (OH stretch), 1,547.47 (C=C stretch), 3,227.35 (NH stretch), 2,995.45 (C-H stretch) ; 1H NMR: (δ) 9.9 (s, 1H, N=CH), 8.4 (s, 1H, NH-N=), 7.8 (d, 2H, Ar-H), 7.5 (m, 2H, Ar-H), 7.4 (m, 3H, Ar-H), 6.9 (m, 2H, Ar-H), 5.2 (s, 1H, OH).
Analytical data for compound AR2
M.P.(°C): 211–214; Yield: 72%; IR (cm−1): 1,720.13 (C=O stretch), 1,625.98 (C=N stretch), 3,636.48 (OH stretch), 3,279.74 (NH stretch), 3,035.96 (Aromatic CH stretch), 1,521.84 (Aromatic C=C stretch), 671.23 (C-Cl stretch), 2,961.45 (C-H stretch), 630.42 (CH Rocking), 1,148.46 (C-C stretch); 1H NMR: (δ) 9.1 (s, 1H, -NH-N=), 8.1 (s, 1H, -N=CH-), 7.8 (d, 2H, Ar-H), 7.6 (d, 2H, Ar-H), 7.3 (d, 2H, Ar-H), 6.9 (d, 2H, Ar-H), 5.4 (s, 1H, OH).
Analytical data for compound AR3
M.P. (°C): 225–227; Yield: 56.5%; IR (cm−1): 1,706.11 (C=O stretch), 1,665.45 (C=N stretch), 3,715.31 (OH stretch), 3,269.13 (NH stretch), 3,056.34 (Aromatic CH stretch), 1,504.54 (Aromatic C=C stretch), 2,921.32 (C-H stretch), 640.39 (CH Rocking), 1,148.46 (C-C stretch); 1H NMR: (δ) 8.0 (s, 1H, -NH-N=), 8.1 (s, 1H, -N=CH), 7.8 (d, 2H, Ar-H), 7.5 (d, 2H, Ar-H), 7.1 (d, 2H, Ar-H), 6.9 (d, 2H, Ar-H), 5.1 (s, 1H, OH).
Analytical data for compound AR4
M.P. (°C): 170–173; Yield: 55.3%; IR (cm−1): 1,717.68 (C=O stretch), 1,627.99 (C=N stretch), 3,558.27 (OH stretch), 3,237.65 (NH stretch), 1,573.98 (NH band), 3,024.51 (Aromatic CH stretch), 1,544.08 (Aromatic C=C stretch), 1,302.01 (C-O stretch), 3,042.44 (C-H stretch), 659.68 (CH Rocking). 1,023.28 (C-C stretch); 1H NMR: δ 9.2 (s, 1H,-NH-N=), 8.1 (s, 1H,-N=CH-), 7.8 (d, 2H, Ar-H), 7.0 (m, 2H, Ar-H), 6.9 (d, 2H, Ar-H), 6.7 (d, 2H, Ar-H), 5.6 (s, 1H, OH), 3.7 (s, 3H, -OCH3).
Analytical data for compound AR5
M.P. (°C): 247–249; Yield: 67.9%; IR (cm−1): 1,710.70 (C=O stretch), 1,638.00 (C=N stretch), 3,562.60 (OH stretch), 1,560.92 (NH band), 3,081.60 (Aromatic CH-stretch), 1,590.92 (NH band), 1,545.65 (C=C stretch), 636.51 (CH rocking); 1H NMR: δ 9.8 (s,1H,NH), 8.4 (s,1H,OH), 7.8 (d, 2H, Ar-H), 7.7 (d, 2H, Ar-H), 7.6 (d, 2H, Ar-H), 7.3 (d, 2H, Ar-H), 7.1 (d, 2H, Ar-H), 7.0 (s, 1H, OCH3), 6.8 (d, 2H, Ar-H).
Analytical data for compound AR6
M.P. (°C): 177–177; Yield: 87.8%; IR (cm−1): 1,690.68 (C=O stretch), 1,636.67 (C=N stretch), 3,554.41 (OH stretch), 3,277.20 (NH stretch), 3,037.05 (Aromatic CH stretch), 1,540.23 (Aromatic C=C stretch), 1,092.72 (C-O stretch), 2,772.79 (C-H stretch), 659.68 (CH Rocking), 1,033.89 (C-C stretch); 1H NMR: δ 8.0 (s, 1H, -NH-N=), 8.1 (s, 1H, -N=CH-), 7.7 (d, 2H, Ar-H), 7.1 (m, 2H, Ar-H), 7.2 (m, 2H, Ar-H), 6.9 (d, 2H, Ar-H), 5.4 (s, 1H, OH), 3.7 (s, 3H, -OCH3).
 | Table 1. Physical data of synthesized derivatives. [Click here to view] |
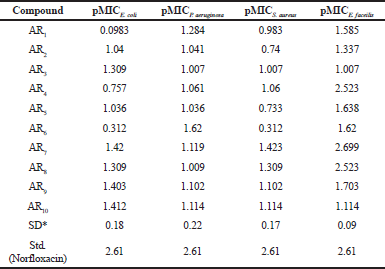 | Table 2. Antibacterial activity of synthesized compounds. [Click here to view] |
Analytical data for compound AR7
M.P. (°C): 164–168 (°C); Yield: 74.9%; IR (cm−1): 1,730.11(C=O stretch), 1,690.25(C=N stretch), 3,675.15(OH stretch), 3,234.45 (NH stretch), 3,024.11 (Aromatic CH stretch), 1,560.45(Aromatic C=C stretch), 1,290.25 (C-O stretch), 2,755.31(C-H stretch); 1H NMR: δ 10.5 (s, 1H, CH), 9.9 (s, 1H, NH), 7.9 (s, 1H, OH), 6.8 (d, 2H, Ar-H), 3.1 (s, 6H, 2OCH3), 2.4 (d, 2H, Ar-H), 2.2 (s, 3H, CH3).
Analytical data for compound AR8
M.P. (°C): 212–215; Yield: 59.8%; IR (cm−1): 1,690.68 (C=O stretch), 1,691.64 (C=N stretch), 3,758.27 (OH stretch), 3,282.99 (NH stretch), 1,576.87 (NH band), 3,026.44 (Aromatic CH stretch), 1,544.68 (Aromatic C=C stretch), 1,105.26 (C-O stretch), 2,779.54 (C-H stretch), 660.65 (CH Rocking), 1,026.17 (C-C stretch); 1H NMR: δ 9.6(s, 1H, NH), 8.4(s, 1H, OH), 7.8(d, 2H, Ar-H), 7.7(d, 3H, Ar-H), 7.6(d, 2H, Ar-H), 7.5 (d, 2H, Ar-H), 3.2(s, 3H, CH3).
Analytical data for compound AR9
M.P. (°C): 125–128; Yield: 48.1%; IR (cm−1): 1,705.10 (C=O stretch), 1,645.74 (C=N stretch), 3,575.45 (OH stretch), 3,258.90 (NH stretch), 3,021.45 (Aromatic CH stretch), 2,650.45 (C-H stretch), 1,510.64 (Aromatic C=C stretch), 675.71 (CH Rocking). 1,019.21 (C-C stretch); 1H NMR: δ 7.8 (d, 2H, Ar-H), 7.7 (d, 2H, Ar-H), 7.4 (m, 2H, Ar-H), 6.9 (d, 2H, Ar-H), 7.0 (s, 1H, -NH-N=), 5.1 (s, 1H, OH).
Analytical data for compound AR10
M.P. (°C): 112–115; Yield: 97.7%; IR (cm−1): 1,720.18 (C=O stretch), 1,685.71 (C=N stretch), 3,658.27 (OH stretch), 3,368.85 (NH stretch), 3,156.44 (Aromatic CH stretch), 1,642.55 (Aromatic C=C stretch), 1,576.87 (NH band), 3,079.54 (C-H stretch), 742.32 (C-Cl stretch), 670.55 (CH Rocking). 1,026.17 (C-C stretch); 1H NMR: δ 7.8 (d, 2H, Ar-H), 7.3 (m, 2H, Ar-H), 7.2 (m, 2H, Ar-H), 6.9 (d, 2H, Ar-H), 7.0 (s, 1H, -NH-N=), 5.1 (s, H, OH), 1.3 (s, 3H, -CH3)
Antibacterial evaluation
In E. coli, compounds AR7 (pMIC = 1.420) and AR10 (pMIC = 1.412); in P. aeruginosa, compounds AR1 (pMIC = 1.62) and AR6 (pMIC = 1.284); in S. aureus, compounds AR7 and AR8 and in E. faecilis, compounds AR4, AR7, and AR8 were emerged as the most active one.
From antibacterial studies, compound AR7, Nʹ-(3,4,5-trimethoxybenzylidene)-4-hydroxybenzohydrazide in case of E. coli, S. aureus, and E. faecilis and compound AR6, Nʹ-(3-methoxybenzylidene)-4-hydroxybenzohydrazide in case of P. aeruginosa were emerged as the most active compound indicating that methoxy group (e-donating group) on benzene ring is essential for antibacterial activity (Emami et al., 2008). All compounds show good antibacterial property which may be due to the presence of benzylidene/1-phenyl-ethylidene which imparts lipophillicity, a parameter responsible for penetration of molecules across the microbial membrane. In case of E. coli, the compound AR10 containing electron withdrawing chlorine group was also found effective which confirmed the fact that different structural requirements are requisite for activity against different microorganisms (Sortino et al., 2011). The above facts are summarized in Figure 1.
Antioxidant evaluation
To verify the fact that antibacterial compounds also possess anti-oxidant activity, the synthesized compounds were evaluated and it was observed that compound AR10 possesses maximum anti-oxidant activity followed by compounds AR3 and AR7 as governed by hydrogen peroxide scavenging activity method, whereas in DPPH scavenging activity, compound AR8 possesses maximum antioxidant activity followed by compound AR9 > AR10. All these compounds show even better antioxidant activity than reference compound Ascorbic acid as depicted by their IC50 (Table 3; Fig. 2a and b)
The high activity of compounds AR2 and AR10 can be explained by the fact that halogens like chloro enhance antioxidant activity due to its redox activity through one electron transfer mechanism (Bala et al., 2012; Dudhe et al., 2013; Hossain et al., 2009). The high activity of compounds AR3, AR7, AR6, AR8, and AR9 can be explained by the fact that methoxy and alkyl/aryl enhanced the stabilization of the generated radical during oxidation (Hadi et al., 2013). There is no correlation between results obtained by both methods, which governs that completely different mechanisms are involved in these two anti-oxidant determination methods.
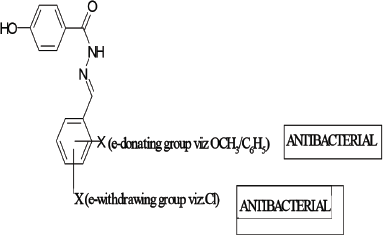 | Figure 1. SAR studies. [Click here to view] |
 | Table 3. IC50 of synthesized derivatives. [Click here to view] |
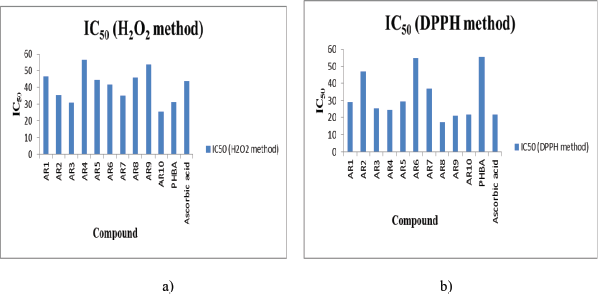 | Figure 2. (a and b) IC50 of synthesized compounds (H2O2 and DPPH method). [Click here to view] |
CONCLUSION
A series of 4-hydroxy-Nʹ-[(1E)-substituted-phenylmethylidene] benzohydrazide derivatives were synthesized and evaluated for its antibacterial and anti-oxidant activity. Compound AR7 was found most potent antimicrobial against E. coli, S. aureus, E. faecilis, and and compound AR6 against P. aeruginosa which might be due to the presence of methoxy group (electron donating group) on benzene ring indicating the importance of electronic parameters in governing potency. Compounds AR10 and AR8 show maximum anti-oxidant activity when governed by hydrogen peroxide and DPPH scavenging activity, respectively, signifying different mechanisms are involved in antioxidant determination.
ACKNOWLEDGMENT
The authors would like to thank the M. M. College of Pharmacy, M. M. (Deemed to be University), Mullana, Ambala for providing support and facility to carry out research.
CONFLICT OF INTEREST
The authors declared no conflict of interest.
FUNDING
None.
REFERENCES
Abouzeed YM, Zgheel F, Elfahem AA, Almagarhe MS, Dhawi A, Elbaz A, Hiblu MA, Kammon A, Ahmed MO. Identification of phenolic compounds, antibacterial and antioxidant activities of raisin extracts. Open Vet J, 2018; 8(4):479–84. CrossRef
Ahmad Al, Ahmad E, Rabbani G, Haque S, Arshad M, Khan RH. Identification and design of antimicrobial peptides for therapeutic applications. Curr Protein Pept Sci, 2012; 13(3):211–23. CrossRef
Alam M, Ali R, Marella A, Alam T, Naz R, Akhter M, Shaquiquzzaman M, Saha R, Tanwar O, Hooda J. Review of biological activities of hydrazones. Indonesian J Pharm, 2012; 23(4):193–202.
Bala S, Uppal G, Kajal A, Kamboj S, Sharma V. Hydrazones as promising lead with diversity in bioactivity-therapeutic potential in present scenario. Int J Pharm Sci Rev Res, 2013; 18(1):65–74.
Bala S, Uppal G, Kamboj S, Saini M. Therapeutic review exploring antimicrobial potential of hydrazones as promising lead. Der Pharma Chemica, 2011; 3(1):250–68.
Bala S, Uppal G, Kamboj S, Saini V, Prasad DN. Design, characterization, computational studies and pharmacological evaluation of substituted-N′-[(1E) substituted phenylmethylidene] benzohydrazide analogs. Med Chem Res, 2012; 21(11):1–13. CrossRef
Benslama A, Harrar A, Gul F, Demirtas I. Phenolic compounds, antioxidant and antibacterial activities of Zizyphus lotus L. leaves extracts. Nat Prod J, 2017; 7(4): 316–22. CrossRef
Cappucino JG, Sherman N. Microbiology—a laboratory manual. Addison Wesley, Redwood City, CA, 1999.
Chaudhary J, Rajpal AK, Judge V, Narang R, Narasimhan B. Preservative evaluation of caprylic acid derivatives in aluminium hydroxide gel–USP. Sci Pharm, 2008;76(3):533–40. CrossRef
Dudhe R, Sharma PK, Dudhe AC, Verma PK. Synthesis and antioxidant activities of 6- Aryl-3,4-dihydro-1-(tetrahydro-3,4-Dihydroxy-5-(hydroxymethyl)-furan-2-yl)-4-phenyl-pyrimidine- 2(1H)-thione derivatives. Eur Chem Bull, 2013; 2(6):341-47.
Emami S, Foroumadi A, Falahati M, Lotfali E, Rajabalian S, Ebrahimi A, Farahyar S, Shafiee A. 2-Hydroxyphenacyl azoles and related azolium derivatives as antifungal agents. Bioorg Med Chem, 2008; 18:141–6. CrossRef
Fu L, Lu WQ, Zhou XM. Phenolic compounds and in-vitro antibacterial and antioxidant activities of three tropic fruits: persimmon, guava and sweetsop. Biomed Res Int, 2016; 2016:9. CrossRef
Hadi A, Alireza F, Reza K, Karim N, Mostafa RT. Synthesis and investigation of antioxidant activities of 2-benzylidene-3-coumaranones. J Paramed Sci, 2013; 4(2):76–81.
Halliwell B, Aerchabach R, Lologer J, Aruoma OI. The characterization of antioxidants. Food Chem Toxicol, 1995; 33(7):601–17. CrossRef
Harer SL, Rajurkar VG, Patil P, Harer PS, Navale SD, Awuti ST, Sonawane AA. Synthesis, characterization and anti-microbial evaluation of some 2- iodo-N'-[(1E)-substituted phenylmethylidene] benzohydrazide analogues. IJPSDR, 2010; 2(2):134–6.
Hossain MM, Shaha SK, Aziz F. Antioxidant potential study of some synthesized N-heterocycles. Bangladesh Med Res Counc Bull, 2009; 35:49–52. CrossRef
Kapoor A, Dahiya SK. Synthesis and evaluation of 2-aryl substituted benzimidazole derivatives bearing 1,3,4-oxadiazole nucleus for antimicrobial activity. Der Pharmacia Lettre, 2016; 8(12):57–67.
Khatkar A, Nanda A, Kumar P, Narasimhan B. Synthesis, antimicrobial evaluation and QSAR studies of p-coumaric acid derivatives. Arab J Chem, 2017; 10(2):S3804–15. CrossRef
Kumar NA, Chauhan LS. Analgesic and anti-inflammatory potential of hydrazones. J Chem Pharm Res, 2014;6(12):916–34.
Lindahl JF, Grace D. The consequences of human actions on risks for infectious diseases: a review. Infect Ecol Epidemiol, 2015; 5(10):3402. CrossRef
Makki MST, Rahman RMA, Ali OAA. Synthesis of new fluorinated 1,2,4-triazino [3,4-b][1,3,4]thiadiazolones as antiviral probes-part II- reactivities of fluorinated 3-aminophenyl-1,2,4-triazinothiadiazolone. Int J Org Chem, 2015; 5(3):1–12. CrossRef
Manuja R, Sachdeva S, Jain A, Chaudhary J. A comprehensive review on biological activities of p-hydroxy benzoic acid and its derivatives. Int J Pharm Sci Rev Res, 2013; 22(2):109–115.
Marwa AM, Ahmed N. Biological activity of some newly synthesized hydrazone derivatives derived from (dicyclopropylmethylene) hydrazone. Eur Chem Bull, 2018; 7(10):280–7. CrossRef
Narang R, Narasimhan B, Sharma S. Synthesis, antimycobacterial, antiviral, antimicrobial activities, and QSAR studies of nicotinic acid benzylidene hydrazide derivatives. Med Chem Res, 2012; 21(9):2526–47. CrossRef
Neda OA, Denista YY, Anelia TM, Magdalena SKB, Virginia IT, Nadya GHA,Vera AH. Design, synthesis, antioxidant properties and mechanism of action of new N,N′-disubstituted benzimidazole-2-thione hydrazone derivatives. J Mol Struct, 2018; 1165:162–76. CrossRef
Nurkenov OA, Satpaeva B, Schepetkin IA, Khlebnikov AI, Turdybekov KM, Seilkhanov TM, Fazylov SD. Synthesis and biological activity of hydrazones of o- and p-hydroxybenzoic acids. Spatial structure of 5-Bromo-2-hydroxybenzylidene-4-hydroxybenzohydrazide. Russ J Gen Chem, 2017; 87(10):2299–306. CrossRef
Pontiki E, Litina DH. Multi-target cinnamic acids for oxidative stress and inflammation: design, synthesis, biological evaluation and modeling studies. Molecules, 2019; 24(1):12. CrossRef
Popiolek L. Hydrazide–hydrazones as potential antimicrobial agents: overview of the literature since 2010. Med Chem Res, 2017; 26(2):287–301. CrossRef
Rajput AP, Rajput SS. Synthesis of benzaldehyde substituted phenyl carbonyl hydrazones and their formylation using Vilsmeier-Haack reaction. Int J PharmTech Res, 2009; 1(4):1605–11.
Rayam P, Anireddy JS, Polkama N, Allakaa TR, Chepurib K, Mukharjee N. Synthesis and biological activity of novel acyl hydrazone derivatives of 3-(4,5-diphenyl1,3-oxazol-2-yl) propanoic acid as anticancer, analgesic and anti-inflammatory agents. J Paramed Sci, 2015; 9(2):157–64.
Ruch RJ, Cheng SJ, Klannig JE. Prevention of cytotoxicity and inhibition of intercellular communication by antioxidant catechins isolated from Chinese green tea. Carcinogensis, 1989; 10:1003–8. CrossRef
Sapra A, Kumar P, Kakkar S, Narasimhan B. Synthesis, antimicrobial evaluation and QSAR studies of p -hydroxy benzoic acid derivatives. Drug Res, 2014; 64:17–22. CrossRef
Shukla S, Mehta A, John J, Singh S, Mehta P, Vyas SP. Antioxidant activity and total phenolic content of ethanolic extract of Caesalpinia bonducella seeds. Food Chem Toxicol, 2009; 47:1848–51. CrossRef
Singh N, Ranjana R, Kumari M, Kumar M. A review on biological activities of hydrazone derivatives. Int J Pharm Clin Res, 2016; 8(3):162–6.
Soares JR, Dinis TCP, Cunha AP, Almeida LM. Antioxidant activities of some extracts of Thymus zygis. Free Radic Res, 1977; 26:469–78. CrossRef
Sortino M, Garibotto F, Cechinel FV, Gupta M, Enriz R, Zacchino S. Antifungal, cytotoxic and SAR studies of a series of N-alkyl, N-aryl and N-alkylphenyl-1,4-pyrrolediones and related compounds. Bioorg Med Chem, 2011; 19(9):2823–34. CrossRef
Sprong RCA, Winkelhuyzen JC, Aarsman J, van Oirschot T, Vandeer B, VanAsbeck B. Low dose N-acetylcysteine protects rats against endotoxin mediated oxidative stress, but high dose increases mortality. Am J Respir Crit Care Med, 1998; 157: 1283–93. CrossRef
Suzana I, Tutuk B. Synthesis, molecular docking and antimicrobial of N’-benzylidene-4-hydroxybenzohydrazide and N’-(4-methoxybenzylidene)-4-hydroxybenzohydrazide. RJPBS, 2017; 8(2):1354–61.
Velika B, Kron I. Antioxidant properties of benzoic acid derivatives against superoxide radical. Free Radical and Antioxidants. 2012; 2(4):62–7. CrossRef
Venkatesh T, Bodke YD, Kenchappa R, Telkar S. Synthesis, antimicrobial and antioxidant activity of chalcone derivatives containing thiobarbitone nucleus. Med Chem, 2016; 6: 440–8. CrossRef
Verma G, Marella A, Shaquiquzzaman M, Akhtar M, Ali MR, Alam MM. A review exploring biological activities of hydrazones. J Pharm Bioallied Sci, 2014; 6(2):69–80. CrossRef