INTRODUCTION
Poor membrane permeation is one of the major governing factors for incomplete oral bioavailability of drugs (Aungst 1993; Savla et al., 2017). About 40% of new chemical entities developed in the pharmaceutical industry and more than 80% of drug candidates in research and development pipeline fails because of solubility problems. At present, about 40% of an immediate release oral drugs in the market are practically insoluble (Kawabataa et al., 2011; Savjani et al., 2012). The solubility and permeability of drug molecule can be correlated with its absorption profile.
Permeability through the gastrointestinal tract is the rate-limiting step for delivering macromolecules and very polar compounds. Poor membrane permeability of drug is attributed to certain physicochemical properties like low octanol/aqueous partitioning, highly polar surface area, high molecular mass, substantial number of hydrogen bonding functional groups, etc., or efflux of drug back into intestinal lumen due to presence of secretory transporters which may include P-glycoprotein (P-gp) and possibly others (Aungst, 2000). In addition to these, as per “Lipinski’s rule of 5,” if the calculated log P of the drug is more than 5 and the molecular mass is more than 500, then that drug has poor absorption or permeation (Lipinski et al., 1997). For oral and intestinal absorption of the drug, the ideal value of log P is 1.35–1.8. Negative value means the drug is more hydrophilic in nature, and thus poorly permeable and bioavailable (Kokate et al., 2008). Poorly permeable and bioavailable drugs remain sub-therapeutic as a given dose of drug never reaches to systemic circulation or produces its biological effect after frequent high-dose administration. In such cases, dose escalation would be required which may lead to gastrointestinal toxicity, and thus a reduction in patient compliance. Also, it is difficult to formulate drug product with a high-drug dose (Dudhatra et al., 2012; Kawabataa et al., 2011). Therefore, there is an increasing medical interest and need to enhance the permeability of drugs especially, which are (1) rarely available, (2) administered for a longer duration, (3) toxic, and (4) expensive. Thus, the major challenging task to pharmaceutical scientists lies with improving the physicochemical properties of poorly permeable drug molecule which are difficult to change. To overcome this, permeability enhancers can be added externally to enhance the permeation transiently.
The pre-treatment of drug with natural and safe herbal compounds such as piperine, quercetin, curcumin, naringin, genistein, glycyrrhizin, sinomenine, ginger, carumcarvi, capsaicin, campul, cow urine distillate, allicin, lysergol, etc., possessing permeability, and bioavailability enhancing activity has gained a great interest in oral delivery of drugs and opens up new horizon in the pharmaceutical and healthcare sector (Dudhatra et al., 2012; Kesarwani et al., 2013). These bioenhancers act by either inhibition of drug metabolizing enzymes and suppression of first-pass metabolism (Atal et al., 1985; Bhardwaj et al., 2002; Reen et al.,1993), reduction of gastrointestinal transit (Bajad et al., 2001), cholagogues effect (Majeed et al., 1996), increasing gastrointestinal blood supply and reducing hydrochloric acid secretion (Annamalai and Manavalan, 2000), stimulation of enzyme activity of γ-glutamyltranspeptidase to enhance uptake of amino acids (Johri et al., 1992), modifications in gastrointestinal tract epithelial cell membrane permeability (Khajuria et al., 2002) and/or thermogenic and bioenergetics properties (Majeed et al., 1996). Nowadays, to improve oral drug delivery, use of P-gp inhibitors is a need of time (Pan et al., 2002). These natural bioenhancers would be expected to enhance the bioavailability of drugs with their reduction of dosing frequency and toxicity (Godugu et al., 2014). Thus, considering the beneficial effects of these bioenhancers, it was thought worthwhile to investigate permeability characteristics as well as the anticancer activity of berberine chloride in presence of bioenhancer quercetin.
Berberine is a natural isoquinoline alkaloid having diverse pharmacological actions like hypolipidemic, anti-inflammatory, antiretroviral, hypoglycemic, antimalarial, antiarrhythmic, antiproliferative, antineoplastic, and antisecretory activity (Holy et al., 2009; Mittal et al., 2014; Pan et al., 2012; Tan et al., 2011; Taylor and Baird 1995; Wang et al., 2018). It has gained considerable attention due to its variety of bioactivities, low toxicity, and cost-effectiveness. Recently, it has been reported that the potential anticancer activity of berberine is due to its regulation of glucose and lipid metabolism in cancer cells. Despite its potential anticancer activity, it has poor intestinal absorption due to epithelial membrane protein P-gp which functions as an efflux pump and therefore its use has been restricted greatly (Pan et al., 2002). It has been reported that after oral administration in humans, berberine shows extremely low and variable blood concentrations (Huaa et al., 2007; Wang et al., 2000) having bioavailability less than 1% (Liu et al., 2010). In clinical conditions, high dose (up to 1.5 g/day) is needed that may lead to adverse gastrointestinal effects (Zhang et al., 2008).
Besides, after oral administration, very low blood concentration of berberine may be due to certain pharmacokinetic causes: (1) enzymes like cytochrome P450 and UDP- glucuronosyl-transferases catalyzed metabolism in the gut and/or liver; (2) excretion of major portion of drug to intestinal lumen, bile and urine (as a substrate of certain efflux transporters) and entero-hepatic circulation; (3) poor absorption (due to some unique physicochemical properties like poor aqueous solubility and dissolution); and (4) predominant tissue distribution (Liu et al., 2010; Zhang et al., 2013). Presence of P-gp efflux pump-mediated excretion of berberine to the gastrointestinal lumen, bile, and urine leads to very low absorption and variable plasma concentration after its oral intake.
It has been hypothesized that co-administration of berberine chloride with bioenhancer might enhance its permeability, plasma concentration, biological actions, and minimize its gastrointestinal adverse effects (Fan et al., 2013).
Various studies have been reported on enhancement of bioavailability of berberine chloride in the presence of bioenhancers, such as use of P-gp inhibitors cyclosporin A, verapamil, etc. (Pan et al., 2002), by spray dried mucoadhesive microparticle formulation (Godugu et al., 2014), chitosan – sodium alginate nanoparticles of berberine hydrochloride to enhance aqueous solubility (Mujtaba et al., 2017), nanoparticles of berberine using different techniques and methods of precipitation of nanosuspension (Sahibzada et al., 2018), use of different types of nano carriers for encapsulation of berberine (Mirhadi et al., 2018), effect of tocopheryl polyethylene glycol succinate (TPGS) as absorption enhancer (Chen et al., 2011), effect of lysergol as bioenhancer (Patil et al., 2013), co-administration with oryzanol (Pan et al., 2012), absorption enhancement by sodium caprate and sodium deoxycholate in rats (Fan et al., 2013), effect of beta-cyclodextrin on intestinal absorption (Zhang et al., 2013), and effect of chitosan and its salt on intestinal absorption in rats (Chen et al., 2012). All the above reports suggested that the presence of bioenhancer yielded a positive effect on permeability, as well as the bioavailability of berberine.
However, none of the experiments were reported for optimization of ex vivo permeability characteristics of berberine chloride in presence of quercetin on goat intestine using Franz diffusion cell. In the present work, a 32 full factorial design was used to optimize the effect of quercetin on the permeability of berberine chloride by pretreatment process. The number of trials has been reduced with the factorial design approach in order to obtain the maximum information on the permeability properties of berberine chloride. The empirical model equation developed through a factorial design approach can be used to characterize the response as a function of the different independent variables. The anticancer activity of optimized batch was performed on different cell lines subsequently.
MATERIALS AND METHODS
Materials
Berberine chloride was provided as a gift sample from Indo German Alkaloids, Mumbai, India. Quercetin was purchased from High Media Laboratories Pvt. Ltd. India. Double distilled water was used for experimental procedures along with chemicals of analytical reagents grade. Goat intestine was obtained within 1 hour from local slaughterhouse after killing of the goat (Garg et al., 2011).
Preparation of receptor fluid
Phosphate buffer of physiological pH 7.4 was prepared as per Indian Pharmacopoeia Monograph and used as a receptor fluid.
Preparation of goat intestine
The ex vivo permeability study was performed using freshly excised goat intestine in phosphate buffer pH 7.4 as goat jejunum is a reliable predictor of oral absorption in humans (Garg et al., 2011). Freshly cut small intestine tissue of goat cleaned by washing with phosphate buffer pH 7.4. Jejunum part was separated and was cut into area 3.2 cm2 and thickness 500–600 μm. Tissue was kept alive by oxygen supply with an aerator and phosphate buffer pH 7.4 up to 1 hour. Pretreatment of goat intestine with three different concentrations of quercetin was done at three different time period, i.e., for 30, 45, and 60 minutes.
Preparation of control
Berberine chloride (10 mg) was used as a control for the present study (Sample code Bo).
Preparation of test sample
Experimental plan for pretreatment study, factors, and levels from the 32 factorial design by response surface analysis is given in Table 1. Dose of berberine chloride was kept uniform (10 mg) all over the study.
Experimental design for optimization
A full 32 factorial design approach was used to study the effect of independent variables on ex vivo permeability characteristics of berberine chloride. In this approach, two factors were studied at three different levels and nine possible combinations were examined experimentally. The two independent variables (factors) studied were concentration of quercetin (X1) and time of pretreatment of the intestine with quercetin (X2). The % cumulative drug release (% CDR) (Y) was chosen as a dependent variable or response. Preliminary trials were performed in order to select the levels for study along with process variables.
Response surface analysis
The optimization of batches by response surface methodology was carried out using STATEASE (design expert, version 9.0.4.1) software. Two factors along with three levels design were used because it was suitable for optimizing permeability parameters by exploring the quadratic response surfaces and constructing second order polynomial model. Based on the model analysis, R2 analysis, lack of fit, and predicted residual sum of squares (PRESS) for measured response % CDR, polynomial equation involving individual factors was selected. A quadratic equation generated by the design was used to fit the surface in the following term.
Where Y—response, b0—intercept, b1–b5—regression coefficients, X1 and X2—individual effects, X12 and X22—quadratic effects; X1X2—interaction effect. To determine the significance of the model (p < 0.05), one-way analysis of variance (ANOVA) was applied. All the responses (% CDR), each in triplicate (n = 3) was expressed as the mean ± standard deviation (SD).
Optimization and validation of model
A contour plot establishes the effect of independent variables on a dependent variable, while ANOVA provided by the software was used to determine statistically significant factors. The best fitting mathematical models were evaluated using comparisons of several statistically significant terms and R2 value provided by Design-Expert software. Batch having a higher value of responses was selected as an optimized batch (O-1) for check-point analysis and has been evaluated for the ex vivo permeability study. The resultant response was quantitatively compared with predicted value and the prediction error was calculated.
Ex-vivo permeability study
In the present investigation, the permeability study of pure berberine chloride in the presence (pretreatment) and absence (control) of bioenhancer quercetin across goat intestinal mucosa (Table 1) was conducted using a Franz diffusion cell in which goat intestinal membrane was tightly clamped with the mucosal side oriented upwards. The receptor chamber having 12 ml capacity and the total area of the intestinal membrane for diffusion was about 3.14 cm2. The receptor fluid was kept at 37°C ± 1°°C stirred at 100 rpm with Teflon-coated magnetic stirring bead. After loading test sample on the donor side, 2 ml aliquot was withdrawn from receptor side at each 30 minutes time intervals maintaining sink condition. Analysis of the sample was carried out by UV-spectrophotometer at 341 nm and cumulative amount permeated was determined (n = 3). Experiment was carried out up to 6 hours. The amount of drug permeated at each time interval was calculated. The data obtained from the ex vivo permeation study were used to derive permeability parameters.
 | Table 1. Experimental plan, factors and levels from the 32 factorial design for pre-treatment study. [Click here to view] |
Data analysis
Experimental data obtained from permeability study for each test and control sample were used to calculate CDR, % CDR (mean ± SD), apparent permeability (Papp), Flux (J), and enhancement ratio (ER) by following standard formulae (Chakraborti et al., 2015; Varma et al., 2014).
Permeability coefficient (apparent permeability)
where VA = volume in acceptor chamber, Area = intestinal membrane surface area, and time = total transport time
Enhancement Ratio (ER) = Papp of combination/Papp of control
In vitro anticancer activity
The cytotoxic effects of optimized PreB3 batch (by pretreatment of cancer cell lines with quercetin for 30 minutes, then treating with berberine chloride), drug berberine chloride and bioenhancer quercetin were determined on various cancer cell lines A459 (Lung Cancer), K562 (Leukemia), and Hela (Cervical Cancer) by 3-(4,5- dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. In a 96-well flat-bottom microplate, cells were seeded in triplicate at a density of approximately 5 × 103 cells/well maintained at 37°C in 95% humidity and 5% CO2 for overnight. Samples of different concentration (400, 200, 100, 50, 25, and 12.5 μg/ml) were treated and cells were incubated for another 24 hours. With the solution of phosphate buffer, cells were washed twice and to each well added 20 μl of the MTT staining solution (5 mg/ml in phosphate buffer solution) and plate were incubated at 37°C. After 4 hours, to each well added 100 μl of dimethyl sulfoxide to dissolve the formazan crystals, and absorbance was recorded at 570 nm using microplate reader (Bhat et al., 2018; Shah et al., 2016). The IC50 of the compound was calculated using graph pad prism version 5.1, and mean cell viability was determined using the following formula:
% cell viability
RESULTS
The results of the ex vivo permeability study of berberine chloride in the presence (pretreatment) and absence (control) of quercetin are shown in Table 2. The effect of different pretreatment time of quercetin on % CDR of berberine chloride is presented in Figures 1–3. The values of % CDR, Papp, J, and ER were also calculated up to 6 hours in cases of all samples as shown in Table 3. The outcome of the model analysis, R2 analysis, and PRESS value for measured response are given in Table 4. Outcome of one-way ANOVA (p < 0.05) used for statistical model evaluation is tabulated in Table 5. As the observed “F-value” was higher than 1.0, which shows significant difference among group means than the expected value. The correlation coefficient (R2) for a response as % CDR shows a high significance and good fit of the models.
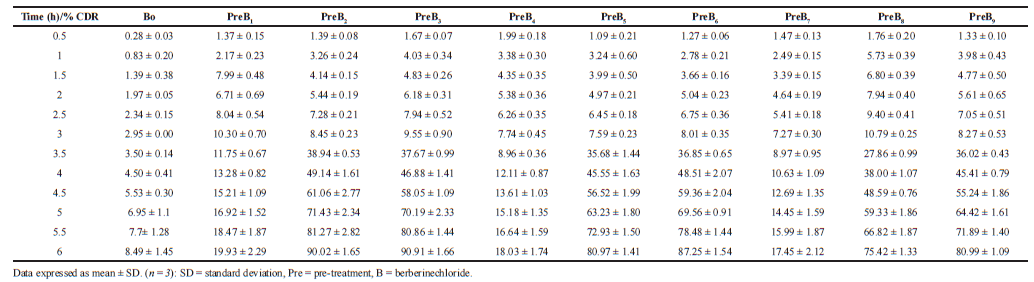 | Table 2. % CDR ± SD of Berberine chloridefrom control and pre-administration study. [Click here to view] |
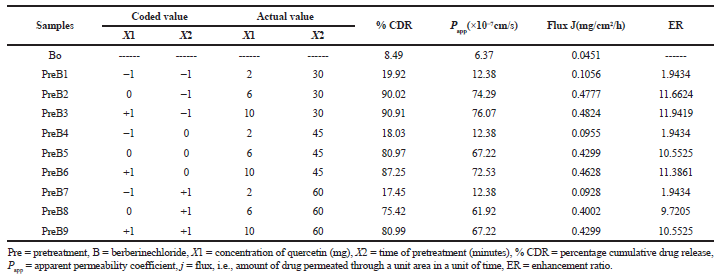 | Table 3. Experimental plan and observed response values with ex vivo permeability profiles from 32 full factorial design. [Click here to view] |
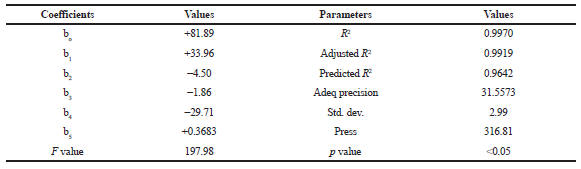 | Table 4. Outcome of model analysis, R2 analysis, and PRESS value for measured response. [Click here to view] |
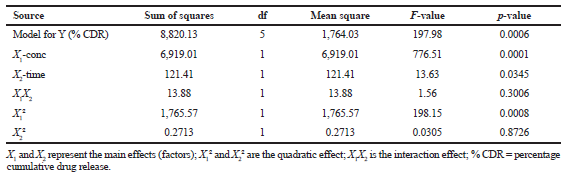 | Table 5. Summary of ANOVA for the response parameters. [Click here to view] |
From the experimental design, the model equation was generated and the response surface data were fitted in Equation (1) and the fitting models for % CDR was given in Equation (2) suggesting an empirical relationship between dependent variable and independent variables in coded unit.
 | Figure 1. Effect of pre-treatment of quercetin for 30 minutes on % CDR. CDR = cumulative drug release. [Click here to view] |
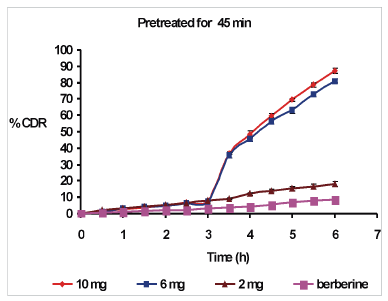 | Figure 2. Effect of pre-treatment of quercetin for 45 minutes on % CDR. CDR = cumulative drug release. [Click here to view] |
 | Figure 3. Effect of pre-treatment of quercetin for 60 minutes on % CDR. CDR = cumulative drug release. [Click here to view] |
Regression equation of the fitted models.
To study the effects of the independent variables on dependent variable, three-dimensional response surface plot (Fig. 4) and corresponding contour plot (Fig. 5) are represented. Permeability study of berberine chloride was optimized for response Y (% CDR) after generating the polynomial equation. Based on the criteria of desirability, the optimal value of response was obtained. Optimization capacity of this model generated was evaluated by selecting an optimized batch (0-1) and ex vivo permeability study was performed. The optimized batch was evaluated for % CDR. Table 6 lists the results of experiments for confirming optimization capability.
In addition to that, IC50 values of compounds in μg/ml and mean cell viability of in vitro anticancer activity of optimized batch, drug berberine chloride, and bioenhancer quercetin on various cancer cell lines K562, A459, and Hela are shown in Tables 7 and 8, respectively.
DISCUSSION
Effect of presence and absence of quercetin on ex vivo permeability of berberine chloride was evaluated by using Franz diffusion cell on goat intestinal membrane up to 6 hours. Berberine chloride (in absence of quercetin-control) has poor membrane permeability showing only 8.49% ± 1.45% CDR. The poor membrane permeability of berberine chloride has various reasons. It was reported that in the chemical structure of berberine, the presence of hydrophobic properties having two methoxy groups and a quaternary ammonium cation leads to low bioavailability and poor stability after its oral administration. Berberine shows high affinity to the gastro-intestinal efflux pump P-gp due to the presence of the cationic group in the structure. Also, the apparent oil-water partition coefficient of berberine (IgPapp= −1.08) indicated low membrane permeability (Li et al., 2017).
Along with the substrate of P-gp, it is a substrate of certain influx organic cation transporters (OCT1 and OCT2), (Nies et al., 2008; Pan et al., 2002). When certain synthetic P-gp inhibitors like cyclosporine, verapamil were co-administered with berberine, there was an enhancement in the absorption of berberine in Caco-2 cells (Pan et al., 2002) which reflects that P-gp may be involved in the efflux of berberine back again into intestinal lumen, leading to poor absorption and thus low bioavailability (Liu et al., 2010).
It was reported that quercetin is a modulator of P-gp (Wang et al., 2004) and can inhibit gastro-intestinal P-gp efflux pump and metabolizing enzyme, CYP3A4 in vitro (Choia et al., 2005; Guengerich et al., 1990; Miniscalco et al., 2002). In the study of oral bioavailability enhancement of diltiazem in rabbits, pre-treated and co-administered with quercetin, it was reported that the bioavailability of diltiazem pretreated with quercetin was increased significantly compared with the control, but no significant improvement in the co-administration case. This might be due to the formation of a complex in the gastrointestinal lumen due to the interaction of quercetin with diltiazem by co-administration of a high dose of quercetin (20 mg). While enhancement in bioavailability of diltiazem was reported when quercetin pretreated for 30 minutes before diltiazem due to early gastro-intestinal absorption of quercetin to inhibit diltiazem metabolizing enzyme CYP3A4 and efflux pump P-gp but not by co-administration (Choia et al., 2005). Similarly, in our ex vivo permeability study on goat intestine, increase in the permeability of berberine chloride during pretreatment with quercetin was found.
During pretreatment study, the regression Equation (2), three-dimensional response surface plot (Fig. 4) and corresponding contour plot (Fig. 5) indicated increase in the concentration of quercetin yielded a positive effect on % CDR, while the increase in pretreatment time by quercetin had a detrimental effect on % CDR. The % CDR was decreased with increase in pretreatment time of quercetin (Table 6). Optimized batch was obtained when goat intestine was pre-treated with quercetin10 mg for 30 minutes giving maximum % CDR of 90.91% ± 1.66%, while minimum value of 17.45% ± 2.12% was obtained at 2 mg quercetin pretreated for 60 minutes as compared with berberine chloride alone (control) which has only 8.49% ± 1.45% CDR. Therefore, increased permeability of berberine chloride during pre-treatment study with quercetin might have resulted from the quercetin, which inhibited the efflux pump P-gp. Briefly, inhibition of efflux pump P-gp by quercetin might be duly responsible for permeability enhancement of berberine chloride.
However, no significant improvement in the in vitro anticancer activity of optimized batch was observed as compared with drug berberine chloride. It was previously reported that quercetin and berberine inhibit survivin and STAT 3 expression (which are responsible for cancer development) and reduce cell viability of gastric cancer cells in a dose-dependent manner. As survivin and STAT 3 are present in lung, leukemia and cervical cancer cells also (Chen et al., 2007; Shukla et al., 2010; 2013; Yoshiyasu et al., 2003), it was observed that berberine showed a strong antisurvivin activity at relatively low doses as compared with quercetin which was found to inhibit survivin and STAT 3 expression only at high concentrations (Pandey et al., 2015). In our study, the concentration of quercetin in the optimized batch was equal to that of berberine chloride. Thus, dose difference might be responsible for non-significant in vitro anticancer activity of optimized batch. As in different types of cancers, constitutive activation of STAT3 and expression of survivin have been widely reported, their linkage may extend to many malignancies and be critical to their pathogenesis (Yoshiyasu et al., 2003).
 | Figure 4. Effect of concentration of quercetin and time on % CDR presented by response surface plot. CDR = cumulative drug release. [Click here to view] |
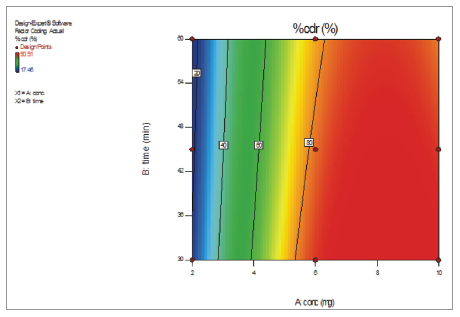 | Figure 5. Effect of concentration of quercetin and time on % CDR presented by contour plot. CDR = cumulative drug release. [Click here to view] |
 | Table 6. Result of experiment for confirming optimization capability. [Click here to view] |
 | Table 7. IC50 Value of Compounds in μg/ml. [Click here to view] |
 | Table 8. Mean cell viability at different concentrations on different cancer cell lines. [Click here to view] |
CONCLUSION
In the present investigation, application of 32 full factorial design approach resulted in rational optimization of PreB3 batch during permeability studies of berberine chloride in presence and absence of bioenhancer quercetin. Based on these data, it could be suggested that 10 mg of quercetin for 30 minutes pretreatment time was optimum to increase the permeability of poorly permeable berberine chloride up to a maximum of 90.91% ± 1.66% CDR. However, anti-cancer cell line studies showed non-significant in vitro anticancer activity of optimized batch as compared with parent drug. It could be concluded that the use of quercetin as a bioenhancer would be beneficial for pretreatment to enhance the permeability and bioavailability of the naturally occurring anticancer drug berberine chloride.
ACKNOWLEDGMENTS
Authors are thankful to Principal, Government College of Pharmacy, Karad, Maharashtra, India for providing laboratory facilities and moral support. Authors are grateful to Maratha Mandal’s NGH Institute of Dental Sciences and Research Centre, Belgaum, Karnataka, India for performing in vitro anticancer activity on cell lines. Authors also express sincere thanks to Appasaheb Birnale College of Pharmacy, Sangli, Maharashtra, India for providing design expert software facility.
CONFLICT OF INTEREST
The authors declare no conflict of interests.
REFERENCES
Annamalai AR, Manavalan R. Effects of Trikatu and its individual components and piperine on gastro intestinal tracts: trikatu: a bioavailable enhancer. Indian Drugs, 1990; 27(12):595–604.
Atal CK, Dubey RK, Singh J. Biochemical basis of enhanced drug bioavailability by piperine: evidence that piperine is a potent inhibitor of drug metabolism. J Pharmacol Exp Ther, 1985; 232(1):258–62.
Aungst BJ. Intestinal permeation enhancers. J Pharm Sci, 2000; 89(4):429–42.
Aungst BJ. Novel formulation strategies for improving oral bioavailability of drugs with poor membrane permeation or presystemic metabolism. J Pharm Sci, 1993; 82(10):979–87.
Bajad S, Bedi KL, Singla AK, Johri RK. Piperine inhibits gastric emptying and gastro-intestinal transit in rats and mice. Planta Med, 2001;67(2):176–9.
Bhardwaj RK, Glaeser H, Becquemont L, Klotz U, Gupta SK, Fromm MF. Piperine, a major constituent of black pepper, inhibits human P-glycoprotein and CYP3A4. J Pharmacol Exp Ther, 2002; 302(2):645–50.
Bhat SS, Revankar VK, Kumbar V, Bhat K, Kawade VA. Synthesis, crystal structure and biological properties of a cis-dichloridebis(diimine)copper (II) complex. Acta Cryst, 2018; C74:146–51.
Chakraborti CK, Sahoo S, Behera PK. Effect of different polymers on in vitro andex vivo permeability of ofloxacin from its mucoadhesive suspensions. Saudi Pharm J, 2015; 23:195–201.
Chen W, Fan D, Meng L, Miao Y, Yang S, Weng Y, He H, Tang X. Enhancing effects of chitosan and chitosan hydrochloride on intestinal absorption of berberine in rats. Drug Dev Ind Pharm, 2012; 38(1):104–10.
Chen, W, Miao YQ, Fan DJ, Yang SS, Lin X, Meng LK, Tang X. Bioavailability study of berberine and the enhancing effects of TPGS on intestinal absorption in rats. AAPS PharmSciTech, 2011; 12(2):705–11.
Chen W, Wang X, Zhuang J, Zhang L, Lin Y. Induction of death receptor 5 and suppression of survivin contribute to sensitization of TRAIL-induced cytotoxicity by quercetin in non-small cell lung cancer cells. Carcinogenesis, 2007; 28(10):2114–121.
Choia JS, Li X. Enhanced diltiazem bioavailability after oral administration of diltiazem with quercetin to rabbits. Int J Pharm, 2005; 297:1–8.
Dudhatra GB, Mody SK, Awale MM, Patel HB, Modi CM, Kumar A, Kamani DR, Chauhan BN. A comprehensive review on pharmacotherapeutics of herbal bioenhancers. Sci World J, 2012; Article ID 637953:1–33.
Fan D, Wu X, Dong W, Sun W, Li J, Tang X. Enhancement by sodium caprate and sodium deoxycholate of the gastro-intestinal absorption of berberine chloride in rats. Drug Dev Ind Pharm, 2013; 39(9):1447–56.
Garg Y, Pathak K. Design and in vitro performance evaluation of purified microparticles of pravastatin sodium for intestinal delivery. AAPS PharmSciTech, 2011; 12(2):673–82.
Godugu C, Patel AR, Doddapaneni R, Somagoni J, Singh M. Approaches to improve the oral bioavailability and effects of novel anticancer drugs berberine and betulinic acid. PLoS One, 2014; 9(3):1–15.
Guengerich FP, Kim HD. In vitro inhibition of dihydropyridine oxidation and aflatoxin B1 activation in human liver microsomes by naringenin and other flavonoids. Carcinogenesis, 1990;11:2275–9.
Holy EW, Akhmedov A, Luscher TF, Tanner FC. Berberine, a natural lipid-lowering drug, exerts prothrombotic effects on vascular cells. J Mol Cell Cardiol, 2009; 46(2):234–40.
Huaa W, Ding L, Chena Y, Gonga B, Heb J, Xub G. Determination of berberine in human plasma by liquid chromatography–electrospray ionization–mass spectrometry. J Pharma Biomed Anal, 2007;44:931–7.
Johri RK, Thusu N, Khajuria A, Zutshi U. Piperine mediated changes in the permeability of rat intestinal epithelial cells. The status of γ-glutamyltranspeptidase activity, uptake of amino acids and lipid peroxidation. Biochem Pharmacol, 1992; 43(7):1401–7.
Kawabataa Y, Wadab K, Nakatanib M, Yamadaa S, Onouea S. Formulation design for poorly water-soluble drugs based on biopharmaceutics classification system: basic approaches and practical applications. Int J Pharm, 2011; 420:1–10.
Kesarwani K, Gupta R. Bioavailability enhancers of herbal origin: an overview. Asian Pac J Trop Biomed, 2013; 3(4):253–66.
Khajuria A, Thusu N, Zutshi U. Piperine modulates permeability characteristics of intestine by inducing alterations in membrane dynamics: influence on brush border membrane fluidity, ultrastructure and enzyme kinetics. Phytomedicine, 2002; 9(3):224–31.
Kokate A, Xiaoling Li, Bhaskara J. Effect of drug lipophilicity and ionization on permeability across the buccal mucosa: a technical note. AAPS PharmSciTech, 2008; 9(2):501–4.
Li YJ, Hu XB, Lu XL, Liao DH, Tang TT, Wu JY, Xiang DX. Nanoemulsion-based delivery system for enhanced oral bioavailability and caco-2 cell monolayers permeability of berberine hydrochloride. Drug Deliv, 2017; 24(1):1868–73.
Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev, 1997; 23(1–3):3–25.
Liu YT, Hao HP, Xie HG, Lai L, Wang Q, Liu CX, Wang GJ. Extensive intestinal first-pass elimination and predominant hepatic distribution of berberine explain its low plasma levels in rats. Drug metabolism and disposition. Am Soc Pharmacol Exp Ther, 2010; 38(10):1779–84.
Majeed M, Badmaev V, Rajendran R. Use of piperine to increase bioavailability of nutritional compounds. United States Patent Number, US005536506A, 1996.
Miniscalco A, Landahl J, Regardh CG, Edgar B, Eriksson UG. Inhibition of dihydropyridine in rat and human liver microsomes by flavonoids found in grapefruit juice. J Pharmacol Exp Ther, 2002; 261:1195–8.
Mirhadi E, Razaee M, Nikouei BM. Nano strategies for berberine delivery, a natural alkaloid of berberis. Biomed Pharmacother, 2018; 104:465–73.
Mittal A, Tabasum S, Singh R. Berberine in combination with doxorubicin suppresses growth of murine melanoma B16F10 cells in culture and xenograft. Phytomedicine, 2014; 21:340–7.
Mujtaba A, Hassan K. Nanotechnology based approach to enhance the potential of chemopreventive agent berberine hydrochloride in cancer therapy. Int J Biol Pharm Allied Sci, 2017; 6(5):1–23.
Nies AT, Herrmann E, Brom M, Keppler D. Vectorial transport of the plant alkaloid berberine by double-transfected cells expressing the human organic cation transporter 1 (OCT1, SLC22A1) and the efflux pump MDR1 P-glycoprotein (ABCB1). Naunyn Schmiedebergs Arch Pharmacol, 2008; 376:449–61.
Pan Y, He S, Liao G, Chen L, Zhang Z. A combination of berberine and γ-oryzanol for hyperlipidemia therapy: an hypothesis. Afr J Pharm Pharmacol, 2012; 6(6):355–8.
Pan GY, Wang GJ, Liu XD, Fawcett JP, Xie YY. The involvement of P-glycoprotein in berberine absorption. Pharmacol Toxicol, 2002; 91:193–7.
Pandey A, Vishnoi K, Mahata S, Tripathi SC, Misra SP, Misra V, Mehrotra R, Dwivedi M, Bharti AC. Berberine and curcumin target survivinand stat3 in gastric cancer cells and synergize actions of standard chemotherapeutic 5-fluorouracil. Nutr Cancer, 2015; 67(8):1295–306.
Patil S, Dash R, Anandjiwala S, Nivsarkar M. Pharmacokinetic study of berberine from rasont and implication of lysergol for its bioavailability enhancement. J Liq Chromatogr Relat Technol, 2013; 36(3):336–48.
Reen RK, Jamwal DS, Taneja SC, Koul JL, Dubey RK,Wiebel FJ, Singh J. Impairment of UDP-glucose dehydrogenase and glucuronidation activities in liver and small intestine of rat and guinea pig in vitro by piperine. Biochem Pharmacol, 1993; 46(2):229–38.
Sahibzada MUK, Sadiq A, Faidah H, Khurram M, Amin MU, Haseeb A, Kakar M. Berberine nanoparticles with enhanced in-vitro bioavailability: characterization and antimicrobial activity. Drug Design Dev Ther, 2018; 12:303–12.
Savjani KT, Gajjar AK, Savjani JK. Drug solubility: importance and enhancement techniques. Int Scholarly Res Netw Pharm, 2012; Article ID 195727:1–10.
Savla R, Browne J, Plassat V, Wasan K, Wasan E. Review and analysis of FDA approved drugs using lipid-based formulations. Drug Dev Ind Pharm, 2017; 43(11):1743–58.
Shah T, Joshi K, Mishra S, Otiv S, Kumbar V. Molecular and cellular effects of vitamin B12 forms on human trophoblast cells in presence of excessive folate. Biomed Pharmacother, 2016; 84:526–34.
Shukla S, Mahata S, Shishodia G, Pandey A, Tyagi A, Vishnoi K, Basir SF, DasBC, Bharti AC. Functional regulatory role of STAT3 in HPV16-mediated cervical carcinogenesis. PLoS One, 2013; 8(7):1–13.
Shukla S, Shishodia G, Mahata S, Hedau S, Pandey A, Bhambhani S, Batra S, Basir SF, Das BC, Bharti AC. Aberrant expression and constitutive activation of STAT3 in cervical carcinogenesis: implications in high-risk human papilloma virus infection. Mol Cancer, 2010; 9:282.
Tan W, Li Y, Chen M, Wang Y. Berberine hydrochloride: anticancer activity and nanoparticulate delivery system. Int J Nanomed, 2011; 6:1773–7.
Taylor CT, Baird AW. Berberine inhibition of electrogenic ion transport in rat colon. Br J Pharmacol, 1995; 116(6):2667–72.
Varma VNSK, Maheshwari MN, Navya M, Reddy SC, Shivkumar HG, Gowda DV. Calcipotriol delivery into the skin as emulgel for effective permeation. Saudi Pharm J, 2014; 22:591–9.
Wang YH, Dawn P, Chao L, Hsiu SL, Wen KC, Hou YC. Lethal quercetin-digoxin interaction in pigs. Life Sci, 2004; 74(10):1191–7.
Wang SD, Song BS, Li K. Determination of berberine in decocted liquid from shenshu granules with water by reversed-phase liquid chromatography. Chin J Chromatogr, 2000; 18(3):261–2.
Wang H, Zhu C, Ying Y, Luo L, Huang D, Luo Z. Metformin and berberine, two versatile drugs in treatment of common metabolic diseases. Oncotarget, 2018; 9(11):10135–46.
Yoshiyasu A, Gerald M, Feldman, Tosato G. Inhibition of STAT3 signaling induces apoptosis and decreases survivin expression in primary effusion lymphoma. Blood, 2003; 101(4):1535–42.
Zhang Y, Cui YL, Gao LN, Jiang HL. Effects of beta-cyclodextrin on the intestinal absorption of berberine hydrochloride, a P-glycoprotein substrate. Int J Biol Macromol, 2013; 59:363–71.
Zhang Y, Li X, Zou D, Liu W, Yang J, Zhu N, Huo L, Wang M, Hong J, Wu P, Ren G, Ning G. Treatment of type 2 diabetes and dyslipidemia with the natural plant alkaloid berberine. J Clin Endocrinol Metab, 2008; 93(7):2559–65.